Detection and Identification of Individual Nanoparticles Using Laser-Induced Breakdown Spectroscopy (LIBS)
As the use of nanoparticles in a wide range of applications continues to increase, the need for techniques to analyze these particles also increases. Javier Laserna of the University of Málaga, in Spain, has developed an approach to measure individual nanoparticles in air using optical trapping (OT) combined with laser-induced breakdown spectroscopy (LIBS). Laserna received the 2018 Lester W. Strock award for this development as well as his broader research on analytical methods and instrumentation using LIBS. He recently spoke to us about this work.
As the use of nanoparticles in a wide range of applications continues to increase, the need for techniques to analyze these particles also grows. Javier Laserna of the University of Málaga, in Spain, has developed an approach to measure individual nanoparticles in air using optical trapping (OT) combined with laser-induced breakdown spectroscopy (LIBS). Laserna received the 2018 Lester W. Strock award for this development as well as his broader research on analytical methods and instrumentation using LIBS. He recently spoke to us about this work.
What led you to investigate LIBS for nanoparticles?
Well, to start with, nanoparticles, as everybody knows, are of extreme interest because of the growing number of applications for these objects in many areas of technology. There are only a few analytical techniques for measuring the composition of nanoparticles, and with most of those techniques, it is only possible to analyze nanoparticles in bulk or when they are attached to a surface or suspended in solution. The ability to analyze individual particles is almost nonexistent; only a few techniques are available, and none of them provide the complete analytical composition of the nanoparticle, as far as I know.
LIBS is well known for its ability to provide panoramic spectra-from the ultraviolet (UV) to the infrared (IR)-in a single shot, in bulk materials. But in the case of nanoparticles, we wanted to analyze these extremely light and extremely low-mass particles in a single shot.
So, we tried it and to our surprise, it worked rather well. The signal-to-noise ratio we obtained was very reasonable considering the very low mass of the nanoparticles.
You developed a method for elemental identification of nanoparticles using LIBS that involves optical trapping. How are the optical trapping and LIBS measurement carried out?
One of the most difficult tasks in this work is to isolate an individual nanoparticle for analysis. This is by no means an easy task. For this purpose we used an optical trap. The concept behind optical trapping technology is rather simple. A continuous-wave (CW) laser beam, propagating upwards, is focused on sample nanoparticles in air or in solution (Figure 1a). The radiation pressure created in this nanoparticle medium is able to counteract the gravity forces that are acting on the particles. So, in principle, this optical field is able to trap the particles. However, the trapping is not always easy, particularly when the particles are very small and when the trapping is performed in air. The air currents in the laboratory, for example, tend to disrupt the particles and they fall out of the trap.
So, we created a confined nanoparticle medium by using a cuvette that is commonly used for spectrophotometry. That way, it is possible to keep the particles inside the trap and free from the air currents. But before you can get to the optical trapping, you have to create an aerosol of the particles you want to analyze. We did that using optical catapulting.
This is done by putting a powder containing nanoparticles on top of a microscope slide. Then we shoot a laser pulse into the nanopowder from under the slide. The shockwave created from the laser pulse beneath the slide creates the nanoparticle aerosol. The aerosol then interacts with the CW laser, which is sent colinearly with the catapulting beam, propagating upwards through the microscope slide. This CW laser is tightly focused by a microscope objective, which is a 20x laser objective with a numerical aperture of 0.4. All of this is done inside the cuvette to keep the nanoparticles from blowing away (Figure 1b).
Figure 1: A schematic of the optical catapulting and optical trapping processes developed by Javier Laserna and his team at the University of Málaga to trap individual nanoparticles for analysis by laser-induced breakdown spectroscopy (LIBS): (a) macro view of apparatus for optical catapulting, trapping and measurement of nanoparticles. (b) close-up of apparatus showing trapped nanoparticle in cuvette (with inset image of apparatus).
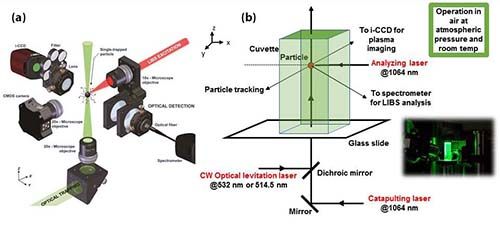
The aerosol created in the cell is highly dispersed, so we have to wait for the nanoparticle to deposit by gravity. Usually, after 3 to 5 minutes, the particle is trapped; the exact time depends on factors such as particle size, particle geometry, and the optical properties of the particle, but most particles are trapped within about 5 minutes.
In the optical trap it is possible to capture a cluster of particles rather than a single particle. So, we repeat measurements several times until the signal has a low range S/N. This measurement response indicates that the particles creating this signal are individual particles.
We can modify the trapping beam to make the particles go up or down in the trap by changing the power of the trapping laser. We can manipulate the particles so that particles that are too large fall outside the trap. Also, we sometimes observe strings of particles in the trap. We manipulate the laser system so that we end up with only one particle in the trap.
The next thing that is important is to have the particle in a precise position in space so that the nanoparticle can be effectively interacted with by the LIBS excitation beam, which is shot perpendicular to the particle trapping beam. This analyzing laser is a moderate-power laser (200 millijoules per pulse), with excitation wavelength set at 1064 nm. We send a single shot of this laser at the nanoparticle that is trapped. It is important that the nanoparticle targeted is precisely located where laser is going to hit. That way, we can measure the spectrum of the particle. If the nanoparticle is not in that precise spot (at 0,0,0 coordinates), the S/N is nil and we don’t see any signal from the analyte. The only thing we see in that case is the spectrum of air.
Then what we observe in the spectra is the mixture of the spectra of the nanoparticle plus the spectrum of air. So how do we get rid of the air?
There are several strategies we can use. The first is to delay integration of the signal by several microseconds. In this way, many of the ionic spectral lines of nitrogen and oxygen in particular are reduced, virtually to the point of disappearing, and so then we have only the weak ionic lines of air-nitrogen of oxygen-and the spectral lines belonging to the nanoparticle. Some atomic lines of oxygen and nitrogen are still present but they can be resolved from the analyte lines by our spectrometer.
The second strategy is to focus the analytical beam a little ahead of the position of the particle.
The most intense lines of air are a little bit off axis of where we are observing the signal. The plasma of air and the nanoparticle are both observed simultaneously. We then, through data analysis, discriminate between the air and particle signals.
This is a complex process, but the important thing is that we are able to discriminate the plasma of air from the plasma of the particle.
Your method enabled detection of nanoparticles smaller than 100 nm-in other words, in the attogram (10–18 gram) regime. What challenges did you have to overcome to achieve detection of particles that small?
At first, we were a bit surprised that this technology, LIBS, could detect such tiny particles. We had heard many times that one of the barriers to wider adoption of LIBS is low sensitivity. We have been working with LIBS for many years, so we knew that what is meant by low sensitivity is low sensitivity when analyzing trace elements in a matrix. For example, in solid materials, such as when we are analyzing an alloy with one element at trace level, LIBS can rarely achieve a limit of detection (LOD) below the low ppm level.
However, the ability to analyze signals from monoelemental materials, like pure copper particles, is much improved and lower LODs than ppm can be achieved.
So, we started investigating this. We started with microparticles in the 2-micrometer size and achieved a reasonable S/N. Then we went down to 100-nm particles and still achieved a very nice signal-not only with metal nanoparticles like nickel particles but also with silica nanoparticles. We analyzed smaller and smaller particles and still obtained an acceptable S/N. So, we learned that LIBS is able to detect extremely low masses when particles are single-phase or single-component particles.
Then, we discovered that we are not limited to analyzing mono-elemental particles. We were able to analyze multi-elemental particles, such as ferrite particles composed of from three to four elements, and we were able to analyze all the components at same time.
So, we kept investigating ever smaller particles, and we were able to analyze 25-nm-sized particles; this is close to the LOD. Ultimately, at this size of nanoparticle, we achieved an LOD on the order of 57 attograms of mass, which is a spectacular tiny size and mass for LIBS and for comparable technologies.
What novel measurements have you been able to achieve with this approach so far?
For the moment we are still doing research work on this approach. We are still working to understand the many processes involved and how to optimize the many variables that affect the system and trying to understand how the signals can be captured in the most efficient manner possible. The solid angle that we use for signal collection in LIBS is very low in comparison with the 4π emitting geometry of a plasma.
We are trying to improve the capture of the photons emitted by the plasma. Also, we are trying to avoid one of the most inefficient steps in spectroscopy. When analyzing emission from a light source with a traditional monochromator or spectrograph, a considerable amount of photons from the source are lost at the entrance slit. This is the very nature of these devices; it is what they are designed to do.
We are trying to avoid this loss of photon signal from traditional monochromator-based measurements by applying a hyperspectral approach to collect the signal from the plasma. By using an appropriate set of filters that collects all the signals from the analyte of interest we expect to capture all the photons and analyze them all.
This research is ongoing, and we still don’t have definitive results. But if this works, we will have much better LODs at a much lower attogram sample mass level.
How could this technique actually be used in a practical application?
To think about this, we are reviewing the sampling efficiency of the approach. As I said before, we can capture one particle about every 3 minutes on average. So, if we need to analyze hundreds of thousands of particles, this rate would be prohibitive, and we could not use this approach as a screening technique for large numbers of particles.
So, what we believe is the main application of our technology is the analysis conditions where we have only very tiny amounts of sample, such as functionalized nanoparticles that are synthesized in very small amounts for biology research. These samples are hard to analyze by other technologies. Most technologies need relatively large amounts of sample. So, when the amount of sample available for analysis is on the order of micrograms, our technology provides a solution.
At the same time, we also intend to increase our sample throughput by analyzing the nanoparticles in a flow. This is another approach that has nothing to do with the optical trapping or catapulting we have been discussing. In this approach, we would create a sample suspension and create an aerosol of microdroplets and a device to dry the sample, so that we can analyze them on the fly, with a kind of time-of-flight (TOF) analyzer using LIBS. We are only starting this work but are hopeful that this will provide much better sample throughput for analyzing single nanoparticles.
What are your next steps in this work?
For now, this is a laboratory technique, but we think this can evolve into a commercial product in the future.
Remember that the need for this technology is growing because there are more and more nanoparticles being used in many types of technologies. That means the need to characterize the chemical properties-not only the optical or magnetic properties-of nanoparticles is increasing. Those chemical properties are very important in many applications. And currently, very few technologies are available to analyze the chemical composition of nanoparticles, so we see opportunity here. So, we plan to protect our work through a patent, and we are preparing a patent application now.
Reference
- P. Purohit, F.J. Fortes, and J.J. Laserna, Angew. Chem. Int. Ed. 56, 14178 –14182 (2017).

Javier Laserna is a professor of analytical chemistry at the University of Málaga, in Málaga, Spain. His current research interests include the use of lasers in chemical analysis, laser-induced plasma spectroscopy; time-of-flight mass spectrometry; secondary ionization mass spectrometry; surface analysis using laser ablation with optical and ion detection, and laser remote chemical analysis. In the 1990s, he succeeded in demonstrating large-scale optics standoff laser induced breakdown spectroscopy for analysis of distant objects. Since then, this technique has been used for the analysis of explosives and in space exploration. Currently, he is a co-investigator of the SuperCam instrument for the NASA Mars 2020 mission. Laserna has participated in several research projects funded by the European Commission’s Framework Programme as both a consortium member and coordinator. He has also been the principal investigator of more than 25 research projects funded by the Spanish central government and the regional goverment of Andalucía. He has published over 300 scientific papers and six books or book chapters and is a co-inventor of seven patents held by the University of Málaga. Laserna was awarded the Spanish Royal Society of Chemistry National Award for Research in Analytical Chemistry in 2009 and the Spanish Society for Applied Spectroscopy National Award for his research career in Applied Spectroscopy in 2010. He is also the 2018 recipient of the Lester W. Strock Award from the Society for Applied Spectroscopy.
Investigating ANFO Lattice Vibrations After Detonation with Raman and XRD
February 28th 2025Spectroscopy recently sat down with Dr. Geraldine Monjardez and two of her coauthors, Dr. Christopher Zall and Dr. Jared Estevanes, to discuss their most recent study, which examined the crystal structure of ammonium nitrate (AN) following exposure to explosive events.
Distinguishing Horsetails Using NIR and Predictive Modeling
February 3rd 2025Spectroscopy sat down with Knut Baumann of the University of Technology Braunschweig to discuss his latest research examining the classification of two closely related horsetail species, Equisetum arvense (field horsetail) and Equisetum palustre (marsh horsetail), using near-infrared spectroscopy (NIR).
An Inside Look at the Fundamentals and Principles of Two-Dimensional Correlation Spectroscopy
January 17th 2025Spectroscopy recently sat down with Isao Noda of the University of Delaware and Young Mee Jung of Kangwon National University to talk about the principles of two-dimensional correlation spectroscopy (2D-COS) and its key applications.
Measuring Microplastics in Remote and Pristine Environments
December 12th 2024Aleksandra "Sasha" Karapetrova and Win Cowger discuss their research using µ-FTIR spectroscopy and Open Specy software to investigate microplastic deposits in remote snow areas, shedding light on the long-range transport of microplastics.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.