Chemical Modifications of Amino-Terminated Organic Films on Silicon Substrates and Controlled Protein Immobilization by FT–IR and Ellipsometry
The formation of organic silane-based thin films on silicon substrates provides a simple opportunity to introduce chemically well-defined thin films at the molecular scale (1).
Joonyeong Kim, Department of Chemistry, Buffalo State, State University of New York.
The formation of organic silane-based thin films on silicon substrates provides a simple opportunity to introduce chemically well-defined thin films at the molecular scale (1). In particular, amino-terminated self-assembling monolayers (SAMs) are of interest because further chemical modifications of amino groups can facilitate the site controlled immobilization of biomolecules via either peptide linkage (e.g., proteins) or phosphoramidite linkage (e.g., oligomer nucleic acids) with an ultimate application to biosensor development (2).
In this note, chemical modifications of surface amino groups on organic thin films followed by the site-selected immobilization of proteins are presented. Amino-terminated organic films were prepared on silicon wafers by self-assembling 3-aminopropyltriethoxysilane (APTES) in anhydrous toluene. Surface amino groups on APTES thin films were chemically converted to N-hydroxysuccinimide (NHS) ester for coupling immunoglobulin G (IgG) via primary amine groups (-NH2) (see Figure 1 (3). Fourier transform infrared spectroscopy with grazing angle attenuated total reflection (FT-IR-GATR) and ellipsometry were employed to monitor modifications of APTES films and protein coupling.
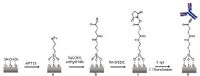
Figure 1: Amino groups in APTES film on silicon substrates were chemically modified to succinylated APTES followed by NHS ester groups, by which IgG is covalently immobilized.
Experimental
P-doped, (100)-oriented silicon wafers (prime grade, 7–21 Ω cm resistivity, 0.5 mm thickness, 5-inch diameter) were cut and cleaned in freshly prepared Piranha solution. APTES films were prepared by incubating silicon wafers and quartz slides in 2% APTES anhydrous toluene solutions for 24 h as described elsewhere (Figure 1a [4,5]). After the deposition, solid substrates were sonicated twice in toluene for 10 min, cured at 100 °C for 24 h, sonicated two times in deionized water for 10 min, and then dried.
Amino groups in APTES films were initially converted to carboxyl groups by incubation in 10 mL THF containing 5 mg/mL succinic anhydride (SA) and 5% triethylamine (TEA) for 4 h (Figure 1b). To introduce NHS ester groups, carboxyl groups on succinylated APTES films were reacted with a mixture of EDC (50 mg/mL) and NHS (5 mg/mL) in 10 mL 0.5 M MES solution for 3 h (Figure 1c [3]). For conjugation via the reaction between NHS ester and primary amine groups, silicon wafers containing NHS ester groups were immersed in IgG solution for 5 h (Figure 1d). After the reaction, unreacted NHS ester groups were deactivated by 0.1 M ethanolamine aqueous solution.
Ellipsometric thickness was measured with a Gaertner L116A automatic ellipsometer equipped with a HeNe laser (632.8 nm) based upon a four-phase model consisting of silicon substrate/SiO2/APTES layer/protein layers (or three-layer model, silicon substrate/SiO2/APTES layer before protein deposition). Optical constants of n = 3.874 and k = 0.016 for Si, n = 1.465 and k = 0 for SiO2, n = 1.424 and k = 0 for and APTES, and n = 1.52 and k = 0 for adsorbed protein were used (6).
FT-IR spectra of intact and derivatized APTES films on silicon wafers in the range of 1900 and 1300 cm-1 were obtained via a grazing angle attenuated total reflection method. A sampling stage equipped with a 60° germanium (Ge) ATR crystal and a high pressure clamp was placed in the sample compartment of a Thermo Scientific Nicolet FT-IR spectrometer (Figure 2). A high pressure swivel clamp 7.8 mm in diameter was used to apply even and constant force (~35 lb) to the sample during FT-IR data acquisition. Each FT-IR spectrum represents the average of 200 scans at 4 cm-1 resolution. A p-polarized infrared beam was used for measurements and the output signal was collected with a deuterated triglycine sulfate (DTGS) detector.

Figure 2: Thermo Scientific Nicolet 6700 with VariGATR.
Results and Discussion
Changes in ellipsometric thicknesses of APTES films after derivatizations and protein immobilization are presented in Table I. The initial thickness of APTES films before chemical modifications was measured to be ~136 Å. After reaction with succinic anhydride (SA) in THF, the thickness increased by ~5 Å. Further increase in the thickness by ~6 Å was observed after the introduction of N-hydroxysuccinimide (NHS) ester groups on the surface via the reaction of COOH-terminated surfaces with NHS in the presence of EDC. Covalent immobilization of intact IgG, followed by deactivation of residual NHS ester groups on the surface using ethanolamine increased the thickness by ~47 Å.
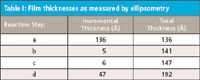
Table I: Film thicknesses as measured by ellipsometry
FT-IR spectra in this region between 1900–1300 cm-1 for APTES films before and after succinylation, after introduction of NHS ester groups, and after coupling with intact IgG are shown in Figure 3. Before modification, two dominating vibrational modes are found in the spectrum of the intact APTES film (Figure 3a). A vibrational mode around 1655 cm-1 arises from the presence of an imine group formed by the oxidation of an amine bicarbonate salt (4,5,8). The other vibrational mode around 1565 cm-1 is attributed to bicarbonated surface amino groups of the APTES film when exposed to air (4, 5, 8).

Figure 3: FT-IR spectra in the range of 1900â1300 cm-1 (a) APTES film produced in dried toluene solutions with the deposition time of 24 h followed by curing at 100 °C for 24 h, (b) after reaction with succinic anhydride, (c) after reaction with NHS/EDC, and (d) after immobilization of intact IgG followed by deactivation of unreacted NHS groups.
After reaction of APTES films with succinic anhydride (SA), the intensity, peak width, and positions of vibrational modes around 1655 and 1565 cm-1 were changed and a new mode was observed around 1405 cm-1 (Figure 3b). These two vibrational modes are typical signatures of amide bonds formed by succinylation of amino groups on APTES films as reported previously (9). Succinylation was also confirmed by the existence of a new vibrational mode around 1405 cm-1 which arises from terminal carboxyl groups of the modified APTES surface (10).
NHS ester groups were introduced on the succinylated APTES surface by reaction with NHS and EDC. The FT-IR spectrum of the surface after reaction reveals several weak features around 1820, 1780, and 1740 cm-1 (Figure 3c). These modes are attributed to the NHS ester groups on the surface. (3)
The FT-IR spectrum of the NHS-functionalized surface after reaction with IgG followed by ethanolamine (Figure 3d) shows that all three mode from NHS ester groups disappeared. In addition, two strong vibrational modes around 1655 and 1545 cm-1 appeared primarily due to amide I and II modes of immobilized IgG.
Summary
Ellipsometry and FT-IR using grazing angle attenuated total reflectance were used to monitor derivatizations of chemically reactive amino groups on APTES thin films followed by covalent immobilization of IgG. Changes in the APTES film thickness after chemical modifications and protein immobilization were confirmed by ellipsometric measurements. FT-IR spectra contain vibrational signatures of these functional groups present in modified APTES films and IgG immobilized on silicon substrates.
References
(1) A. Ulman, Chem. Rev. 96, 1533–1554 (1996).
(2) G. MacBeath, A.N. Koehler, and S.L. Schreiber, J. Am. Chem. Soc. 121, 7967–7968 (1999).
(3) M. Schäferling, M. Riepl, P. Pavlickova, H. Paul, D. Kambhampati, and B. Liedberg, Microchim. Acta 142, 193–203 (2003).
(4) J. Kim, P. Seidler, C. Fill, and L.-S. Wan, Surf. Sci. 602, 3323–3330 (2008).
(5) J. Kim, P. Seidler, L.-S. Wan, and C. Fill, J. Colloid Interf. Sci. 329, 114–119 (2009).
(6) J.D. Le Grange, J.L. Markham, and C.R. Kurkjian, Langmuir 9, 1749–1753 (1993).
(7) J.E. Olsen and F. Shimura, Appl. Phys. Lett. 53, 1934–1936 (1988).
(8) S.R. Culler, H. Ishida, and J.L. Koenig, J. Colloid Interf. Sci. 109, 1–10 (1986).
(9) N. Aoki, M. Nishikawa, and K. Hattori, Carbo. Polym. 52, 219–223 (2003).
(10) B.L. Frey and R.M. Corn, Anal. Chem. 68, 3187–3193 (1996).
Thermo Fisher Scientific
5225-4 Verona Rd., Madison, WI 53711
tel. (800) 532-4752
Website: www.thermoscientific.com
