Coming to a Screen Near You?
The United States Food and Drug Administration (FDA) is using Remote Interactive Evaluations (RIE) to assess regulatory compliance, review submission material, or determine the timing of future inspections. Here, we look at some of the impacts of RIE on GxP laboratories. Although RIE is voluntary, is this an offer that you cannot refuse?
Since the pandemic, changes have occurred in the regulatory oversight of GxP laboratories because of travel restrictions or country access. Instead of an inspection (physical entry to a facility) via remote oversight, assessments and/or evaluations have been used by various regulatory authorities. For example, the United States Food and Drug Administration (FDA) has used Remote Interactive Evaluations (RIE) (1) since 2021 and Remote Regulatory Assessments (RRA) since 2022 (2). For RRA, the FDA requests records and documents to be transferred to them for review, with the process being conducted entirely remotely (2). In some cases, the remote review outcome can be a warning letter and/or an import ban for a company (3–7). In 2024, the FDA provided an update to RRA draft guidance to promote greater consistency in the way RRAs are conducted (8). The FDA has also issued draft guidance on Alternative Tools for assessing drug facilities (9), which references the RRA draft guidance. There is also an agency web page explaining Remote Oversight Tools (10). Any interaction with a facility other than inspection or a record request is considered as RIE (11).
The European Medicines Agency (EMA) has also issued guidance on distant assessments for Good Manufacturing Practices (GMP) (12). These remote assessments and evaluations are not a substitute for inspections, but rather an expansion of regulatory oversight (12).
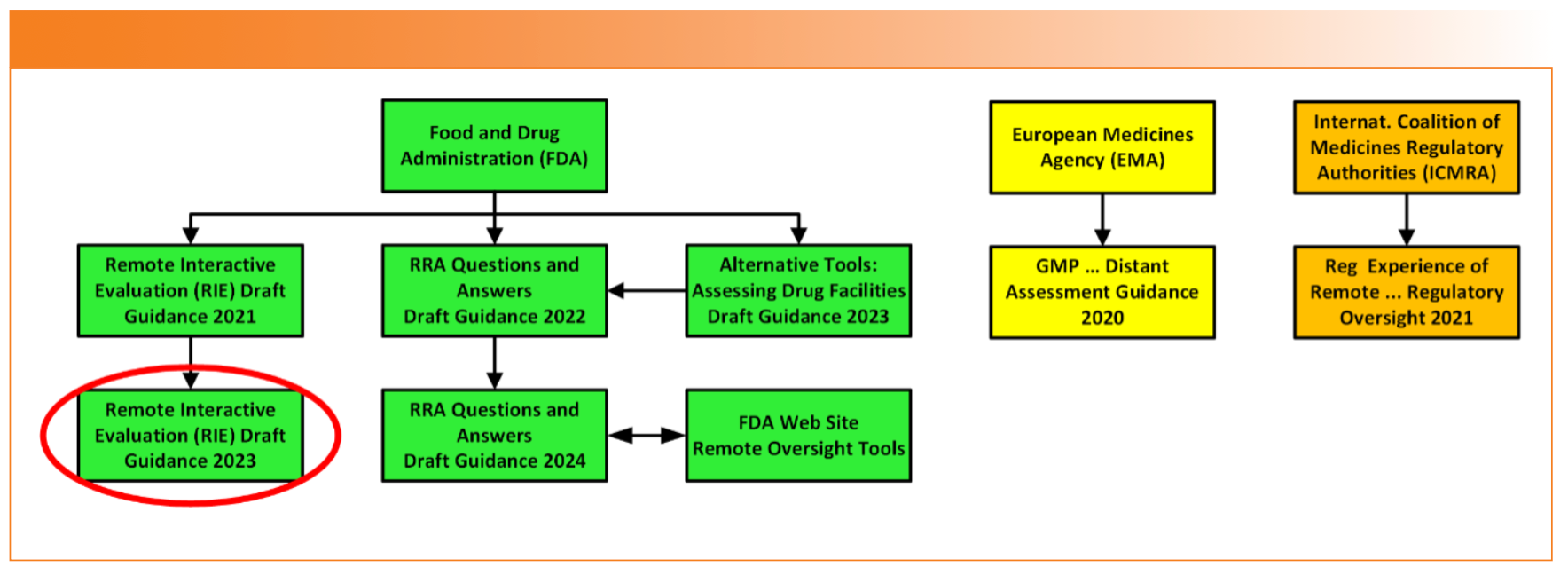
Recently, the FDA updated the draft guidance for Remote Interactive Evaluations (13), and our focus here is on the interpretation of this document for a regulated spectroscopy laboratory. Where there are gaps in the draft guidance, we have added the experience of the International Coalition of Medicines Regulatory Authorities (ICMRA) on remote regulatory oversight (14). The FDA is a member of and a contributor to ICMRA. In addition, some of our personal experiences of remote audits will also be included where appropriate. Post-pandemic, the FDA are continuing to use RRA and RIE for regulatory oversight in some cases. The various sources cited for remote regulatory oversight are shown in Figure 1, but our focus is on the October 2023 RIE draft guidance, circled in red. In this column, the words “investigator” and “inspector” are equivalent in meaning and relative to a person from a regulatory authority conducting on-site or remote regulatory oversight.
FIGURE 1: Sources of Remote Regulatory Oversight.
When is the FDA So Close, Yet So Far Away?
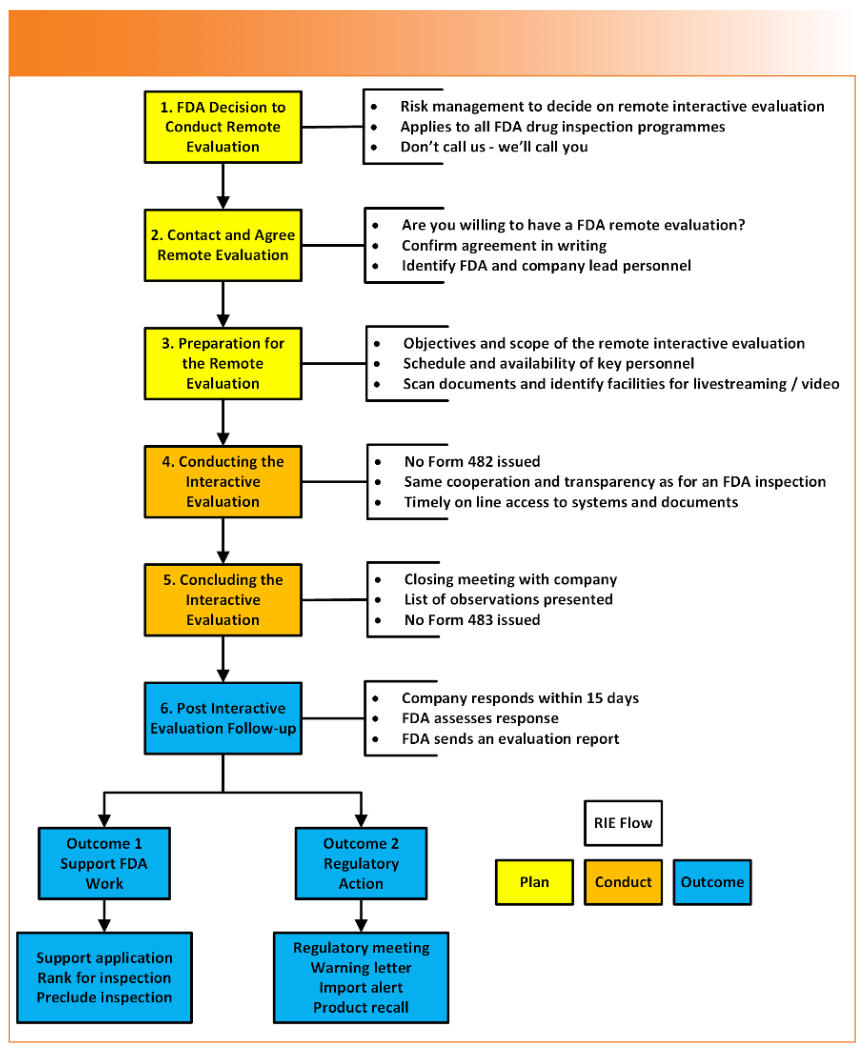
In the next pages, we discuss how RIE could be conducted when evaluating a regulated spectroscopy laboratory and how a laboratory should prepare. The key steps of RIE are shown in Figure 2.
FIGURE 2: FDA Remote Interactive Evaluation Workflow (13).
Facility Selection for a RIE
Will you consider yourself lucky to be selected for RIE? The RIE planning phase is shown in yellow in Figure 2 and described below:
- Have you been good boys and girls? The FDA will not offer RIE to any laboratory with a poor compliance history or data integrity issues. For example, have you received a warning letter or 483 observations for recurrent issues? If so, the FDA does not put your name on the guest list. For the inquisitive, the CDER risk-based site selection model is found in the FDA Manual of Policies and Procedures (MAPP) section 5014.1 (15).
- Don’t call us, we’ll call you: You can’t volunteer for a RIE. The FDA decides and initiates a request to an organization with good compliance history. The guidance states the acceptance of RIE is voluntary, but is this an offer that can’t be refused? If you turn the request down, you could delay a regulatory decision related to the facility inspection or pending drug application. Are you feeling lucky?
- Agree and plan the RIE: Ideally, you should consent to the RIE in writing. Afterwards, the FDA runs a brief meeting to generate the virtual agenda which should cover the schedule, logistics, language, and recording. If the laboratory is within the scope of the RIE, you could have a starring role in the RIE. It is helpful to hold rehearsals to get prepared for the conducting phase (14). We discuss this topic later in this column.
- Schedule: The FDA and the laboratory will agree on the time for RIE meetings, taking into account any time zone differences. ICMRA notes that a remote evaluation takes longer in comparison to an onsite inspection (14). The time needed for the remote tour and documentation review are important elements that should be discussed during the meeting.
- Logistics: The RIE will use FDA video conferencing platforms, and it is important that company IT ensures connection quality and bandwidth. Limitations, sharing methods as livestreaming and/or pre-recorded video, and ways of providing access to the electronic records should be discussed. The RIE guidance briefly outlines the need for direct and encrypted view access to the electronic systems (13); however, per ICMRA experience, if direct access cannot be granted, providing a member of staff to access the system under the direction of the inspector could be a solution (14). For example, how can an investigator view the screen of a standalone spectroscopic system via live streaming? Is there sufficient live streaming resolution to read what is on the screen?
- Language: All documents and discussion will be in English; therefore, a translator will need to be available for the RIE if English is not your native language or if you are not fluent. Although it is not stated in RIE guidance, there is a section on this subject in the RRA guidance lines 417–422 (8). Nevertheless, in February 2024, the US Government Audit Office (GAO) voiced concerns with the FDA’s practice of relying on interpreters provided by the foreign establishment being inspected raises serious concerns about the accuracy of information being gathered (16).
Conducting the RIE
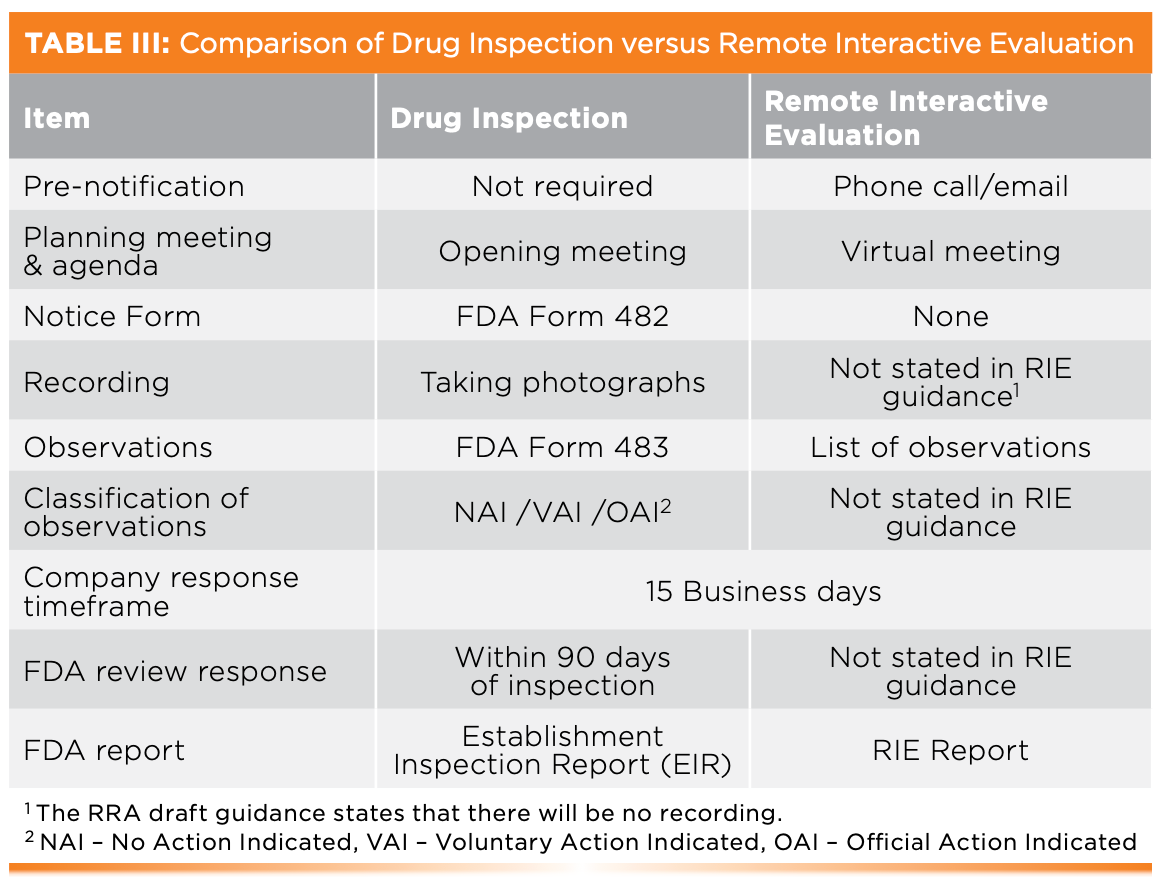
This process is shown in orange in Figure 2. The RIE starts with a virtual opening meeting, and the investigator will work through the agenda. Traditionally, an inspection will start with an investigator presenting a Form 482 Notice of Inspection; however, this does not happen in a RIE (13). Live streaming a remote video tour of a laboratory and record and information review is discussed in the next sections.
Personnel and records are the two fundamental elements that need to be synchronized to build transparency and trust with the investigator. This is the same approach that you would take for an inspection.
- Personnel: Having the right SMEs at the right time, in the right environment, with the right technology. They also need to be trained to handle inspector questions and cooperate fully.
- Records: Availability of the scanned searchable documents and secure access to the electronic files under direction of the investigator. Timely online access to systems and documents needs to be ensured. In terms of the extensive paper-based systems, the ICMRA has found them to be a significant limiting factor for running remote inspection (14).
Lights, Camera, Action?
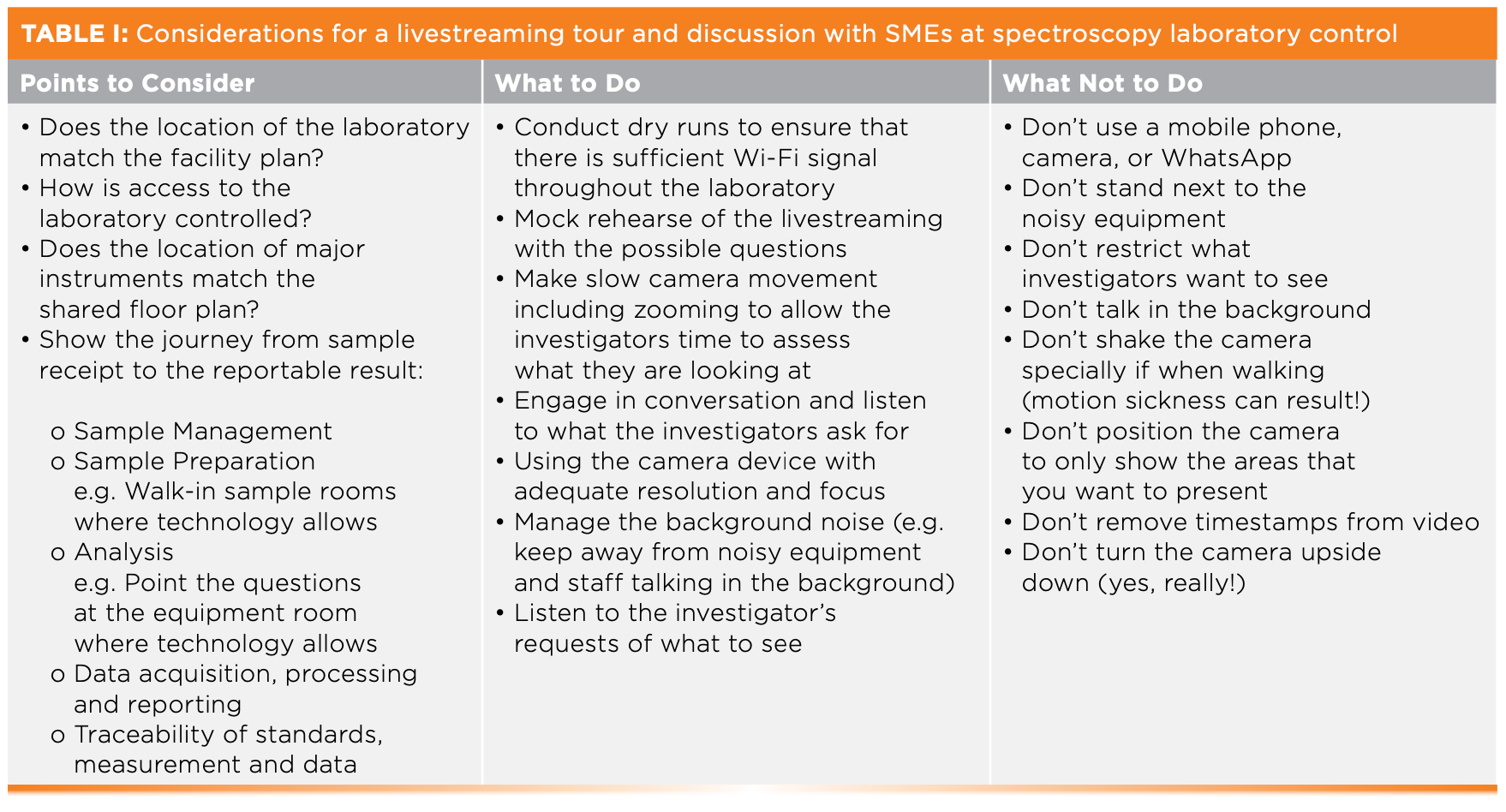
Are you the next Steven Spielberg? One of the most important portions of a RIE is a virtual video laboratory tour. Based on our experience, a video tour must be interactive and filmed with adequate camera resolution. A pre-recorded slide show is unacceptable because it only gives the laboratory’s perspective and may not be what an inspector wants to see. RIE mentions pre-recorded videos (13) may be used, but these suffer from a lack of investigators’ direction. Table I presents our dos and don’ts for livestreaming. One key point is to rehearse the live streaming laboratory tour and to ensure that there is enough bandwidth throughout the laboratory to handle any requests from the investigator. If there is signal drop out, manage an inspector’s expectations at the opening meeting or, better still, set up a WiFi repeater to extend signal coverage. One problem with a remote tour is that an inspector cannot look into wastepaper bins and desk drawers easily.
An Oscar-Winning Performance?
The organization needs to identify laboratory SMEs and appoint trained and experienced staff who know the procedures, spectroscopy instruments, and application software. The assigned team needs to be aware of laboratory processes such as sample receipt and storage, sample preparation, instrument analysis, data generation, retention, and integrity and back-up processes. Additionally, they should be trained in handling inspection questions: if you cannot answer, refer to your supervisor or someone who can. There needs to be an individual documenting what the inspector asks for and if the request has been fulfilled.
Table II presents the points that should be considered when the laboratory is sharing documents; again, this is not exhaustive. Preparation is essential so that you don’t end up with a Golden Raspberry instead of an Oscar-winning performance.
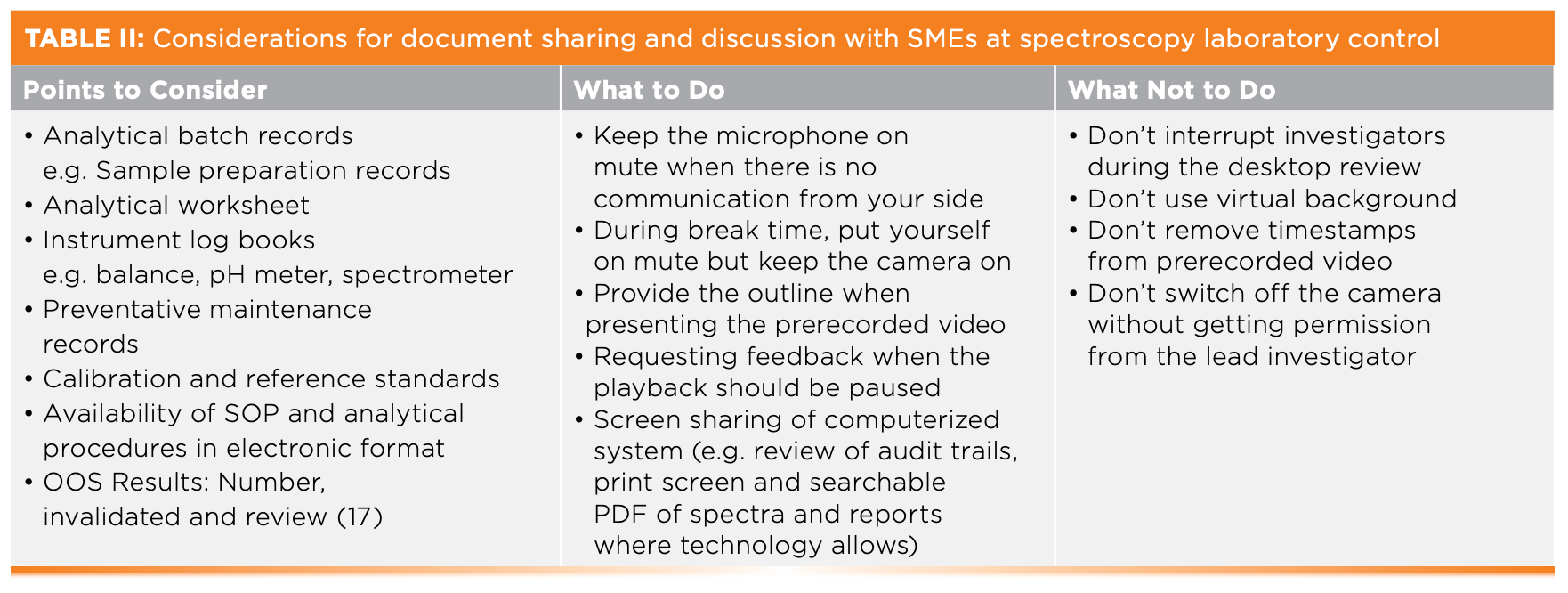
Spectroscopists should be skilled in handling the request for remote document reviews because turnaround time is needed and the review process could take longer in comparison to the inspection, as shared by the ICMRA (14).
A review of spectroscopic records could find that IR identity testing is a subjective process, with analysts making a manual comparison rather than using the instrument’s application’s compare function or a spectral library. This could result in a video replay back in the laboratory looking at the electronic records in the application software. Inexperienced use of application software has been seen in remote audits and is an indication of a laboratory failing to keep current (18,19). Just remember Cahn’s Axiom: when all else fails, read the manual.
Closing Meeting
Will you get a form 483? No, but management gets a list of observations at the close-out meeting.
The close-out session is an opportunity to discuss possible remediation actions. The written response is required to be submitted within 15 business days. The company must treat the violations with the same weight as a 483 form because failure to address them adequately can lead to a warning letter or even an import alert (Figure 2) (3–7). The company gets a copy of the final report after the FDA assesses the response to the RIE observations.
Follow-Up Activities
These are marked in blue in Figure 2. The nature of the observations and how the company responds to them determines how the FDA will react. For example, the RIE finds that there are conflicts of interest or shared user identities on a single standalone spectrometer. There are two options. The first is that the laboratory takes a systematic approach and checks all computerized systems and resolves any similar systems. This may be acceptable to the agency. In contrast, just fixing the system where the problem was identified is an unacceptable approach because there may be other systems where the problem exists.
On the positive side, an outcome of a RIE could be that an on-site inspection is avoided or that a submission is approved. On the negative side, a meeting with the FDA, a warning letter, or an import alert for companies outside of the United States could be received. Instead of an Establishment Inspection Report after an on-site inspection, the organization will receive the RIE report.
What’s Missing from the Draft Guidance?
The draft guidance does not mention the following points:
- Can the FDA or the company record the RIE sessions? This subject is not mentioned in the RIE guidance. However, in the RRA draft, guidance footnote 26 states that the FDA does not intend to record RRAs conducted via livestream, video, or screen sharing; however, FDA may request records (e.g. documents) we review during those sessions (8). As noted earlier, in an inspection, the company uses a person to document what was seen and handle inspector requests.
- There is no mention of the duration of a RIE. However, the ICMRA states that the duration of the remote inspection is longer than an onsite inspection (14).
- Although it is not mentioned in the guidance, laboratories should ensure that the agenda contains a daily wrap up session if the RIE has a duration longer than one day.
- Even though RIE is voluntary, the timeframe for submitting the written consent should be specified.
- A 483 form is formally signed by the inspectors, but for RIE observations, there is nothing stated in the draft guidance.
- 483 observations are classified, as shown in Table III, which can lead to the issuance of a warning letter and/or import alert. However, the draft guidance does not mention how RIE observations will be rated. As RRA has resulted in the issuance of warning letters as shown earlier, can we assume the same approach for RIE?
- The timeline for arranging the briefing meeting, as well as the required time for report distribution and response review, should be defined.
When Is An Inspection Not An Inspection?
When it is Remote Interactive Evaluation! Table III shows the comparison between the Drug Inspection and RIE and highlights the differences between the two. Although a form 483 is not issued at the end of a RIE, both have a list of observations that must be addressed by the laboratory management. RIE is equivalent to an inspection as outcomes could be the same: a warning letter and an import alert for foreign companies.
The view of ICMRA on remote inspections is:
Remote inspections are an enabling tool to maintain at least a minimal regulatory oversight during the pandemic. It is not the view that remote inspections would fully replace an on-site inspection programme (14). This is echoed by the EMA guidance (12).
Conducting effective interviews under remote inspection approaches is not as effective as on-site, as inspectors are not able to assess body language fully and identify evasive and nervous behaviors. Due to the potential lack of spontaneous response when working remotely, this also creates some challenges in knowing how well inspectees know their own procedures and follow them (14).
Similar views about RIE and RRA were voiced at a U.S. Congressional hearing in February 2024, that described alternative inspection methods, such as virtual inspections, record reviews, and reliance on home-country regulators, inadequate to oversee foreign drug operations. “These tools are no substitute for in-person inspections, given the risk of coverup and fraud (16).” A possible way to mitigate some of this concern could be the use of the FDA’s risk-based site selection tool (15).
FDA Strategy to Develop Integrated Remote Tools
RIE is not going away, as a post on the FDA website last year makes clear (20). The FDA is developing Artificial Intelligence (AI) tools/models in four areas: video transcription of voice, translation to English, document and evidence management, and co-working space. If successful, the translation element could overcome the GAO criticism about the FDA relying on translators paid by the inspected company (16). The coworking space portion appears to be a joint project with the University of Maryland to build a system to manage different documents and evidence and share with Agency co-workers. The system consists of three sub-systems: (a) document classifier; (b) video/audio classifier; and (c) an interactive middleware that connects the trained model at the backend and the input at the frontend (20). Let’s see what happens!
Use of a Mixed Reality Device for Inspections
Recently, Baker and others (21) have published their work of a pilot study through using a mixed-reality (hybrid) device to facilitate remote inspections. As preparation, both sides ensured the availability of SMEs and technology platforms and performed practice runs. Two of the authors, who are ex-FDA investigators with a representative from Northeastern University, conducted a one-day mock inspection of a manufacturing facility covering QC, production, and warehouse. Areas captured by the device during a remote facility tour could be pinned and held for future evaluation during the inspection. Accessing the Internet via wireless hotspots was unsatisfactory, especially in production, resulting in poor image quality. Participants from several National Regulatory Authorities, as well as WHO representatives, observed and performed a pilot remote inspection using their own procedures and feedback to the main inspectors. Overall, use of a platform for mixed reality remote inspection is feasible to perform GMP inspections and further testing of improved technology will provide a more seamless experience (21).
Summary
We have discussed Remote Interactive Evaluation, where spectroscopists may find the FDA so close yet so far away! RIE is not a substitute for an on-site inspection, but it is an alternative for laboratories with a good compliance history.
Our column highlights the challenges and advantages for laboratories. The fundamental challenge is around spectroscopic standalone system design and ability to share records and data easily with the investigators. Has the spectroscopist who gets the short straw to be interviewed been trained to handle remote evaluation including investigator’s questions?
RIE should be an opportunity to support timely regulatory decisions and laboratories selected should take advantage of this approach.
Acknowledgments
The authors thank, in alphabetical order, Peter Baker, Chris Burgess, Andrea Chellini, and Andrea Kurz for their critique and constructive comments provided during the preparation of this column. We also appreciate your identification of the additional sources of information.
Mahboubeh would like to thank Bob; his support has been an important part of my journey, and I would like to express my profound gratitude for his mentorship.
References
(1) FDA Guidance for Industry Remote Interactive Evaluations of Drug Manufacturing and Bioresearch Monitoring Facilities During the COVID-19 Public Health Emergency. 2021, Food and Drug Administration:,Silver Spring, MD.
(2) FDA Draft Guidance for Industry Conducting Remote Regulatory Assessments Questions and Answers. 2022, Food and Drug Administration, Silver Spring, MD.
(3) FDA Warning Letter Dr Retter Ec Wladyslaw Retter. 2022, Food and Drug Administration, Silver Spring, MD.
(4) FDA Warning Letter Nantong Furuida Packaging Products Co., Ltd. 2023, Food and Drug Administration, Silver Spring, MD.
(5) FDA Warning Letter Ningbo Poplar Daily-Use. 2024, Food and Drug Administration, Sliver Spring, MD.
(6) FDA Warning Letter Woorilife & Health. 2023, Food and Drug Administration, Silver Spring, MD.
(7) FDA Warning Letter Patcos Cosmetics Pvt. Ltd. 2023, Food and Drug Administration, Silver Spring, MD.
(8) FDA Draft Guidance for Industry Conducting Remote Regulatory Assessments Questions and Answers. 2024, Food and Drug Administration, Silver Spring, MD.
(9) FDA Draft Guidance for Industry Alternative Tools: Assessing Drug Manufacturing Facilities Identified in Pending Applications. 2023, Food and Drug Administration, Sliver Spring, MD.
(10) FDA’s Remote Oversight Tools. 2023. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-references/fdas-remote-oversight-tools (accessed 03-07-2024).
(11) Christensen L.; Chasey. M. Best Practices for Remote Interactive Evaluations and other Alternative Inspection Approaches. in FDA Generic Drug Forum 2023. Food and Drug Administration, Silver Spring, MD.
(12) EMA Guidance Related to GMP/GDP and PMF Distant Assessments. A GMP/GDP Distant Assessment Guidance (Version 1). 2020, European Medicines Agency, Amsterdam, The Netherlands.
(13) FDA Draft Guidance for Industry Remote Interactive Evaluations of Drug Manufacturing and Bioresearch Monitoring Facilities. 2023, Food and Drug Administration, Sliver Spring, MD.
(14) Reflections on the Regulatory Experience of Remote Approaches to GCP and GMP Regulatory Oversight During the COVID-19 Pandemic. 2021.: https://icmra.info/drupal/sites/default/files/2021-12/remote_inspections_reflection_paper.pdf (accessed 03-07-2024).
(15) Understanding CDER’s Risk-Based Site Selection Model, Manual of Policies and Procedures, MAPP 5014.1. 2018, Food and Drug Administration, Silver Spring, MD.
(16) Lawmakers Voice Concerns over FDA’s Foreign Inspection Program. 2024. https://www.raps.org/news-and-articles/news-articles/2024/2/lawmakers-voice-concerns-over-fda%E2%80%99s-foreign-inspec (accessed 03-07-2024).
(17) McDowall, R. D. Data Integrity and Data Governance: Practical Implementation in Regulated Laboratories; Royal Society of Chemistry, 2019.
(18) Facts About the Current Good Manufacturing Practices (CGMPs). 2021. https://www.fda.gov/drugs/pharmaceutical-quality-resources/facts-about-current-good-manufacturing-practices-cgmps (accessed 03-07-2024).
(19) Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community Code Relating to Medicinal Products for Human Use. Official Journal of the European Union 2001, 311, 67.
(20) FDA Analysis and Strategy of Tools to Improve Remote Interactions and Document Management. 2023. https://www.fda.gov/science-research/advancing-regulatory-science/analysis-and-strategy-tools-improve-remote-interactions-and-document-management (accessed 03-07-2024).
(21) Baker, P.; Cathey, T.; Auclair, J. R. Evaluation of a Pilot: Inspection Facilitation and Collaboration Using a Mixed Reality Device. Ther. Innov. Regul. Sci. 2024, 58, 11–15. DOI: 10.1007/s43441-023-00594-2
R.D. McDowall is the director of R.D. McDowall Limited and the editor of the “Questions of Quality” column for LCGC International, Spectroscopy’s sister magazine. Direct correspondence to: SpectroscopyEdit@MMHGroup.com ●

About the Co-Author
Mahboubeh Lotfinia works as a Qualified Person and Quality Partner at F. Hoffmann-La Roche and is trained in GMP/GDP audit execution and CSV (Computerized System Validation). ●

Synthesizing Synthetic Oligonucleotides: An Interview with the CEO of Oligo Factory
February 6th 2024LCGC and Spectroscopy Editor Patrick Lavery spoke with Oligo Factory CEO Chris Boggess about the company’s recently attained compliance with Good Manufacturing Practice (GMP) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Expert Working Group (Q7) guidance and its distinction from Research Use Only (RUO) and International Organization for Standardization (ISO) 13485 designations.