Fast Curing Reaction Monitoring with Dual-Comb Spectroscopy
Special Issues
Fast spectroscopic monitoring is a proven tool in R&D and production environments to gain insight into chemical reactions. This application note showcases how the IRis-F1 dual-comb spectrometer can be used to monitor curing reactions, where it can provide invaluable insight into the performance of coatings and adhesives.
Infrared spectroscopy is in many ways an ideal measurement technique for this application as it is capable of performing non-invasive in-situ measurements that provide direct information about the structure and bonding of the sample. However, many high-performance adhesives possess fast curing rates, and the speed of off-the-shelf spectroscopic techniques is the limiting factor in their analysis.
The IRis-F1 is based on quantum cascade laser (QCL) frequency comb technology, which allows for measurements on microsecond to minute timescales to be carried out.
Experimental
Measurements were conducted with a single-reflection diamond Golden Gate ATR accessory (Specac). The sample is a UV-activated adhesive, which was kindly provided by DELO Industrial Adhesives (Germany). When uncured, it has an infrared band at 1613 cm-1 and a new band at 1638 cm-1 after curing.
The spectrometer was set to a resolution of 4 cm-1 and the acquisition time was 1 ms, with a repetition rate of 40 Hz. Curing was triggered using a mercury lamp (LC8, Hamamatsu), via an optical fiber. After starting the measurement, the lamp is turned on to initiate the curing reaction. For each measurement, a rapid series of measurements spanning before, during, and after curing was taken.

Figure 1: Set-up: IRis-F1 dual-comb spectrometer with ATR GoldenGate for µs/ms reaction monitoring; 40 Hz for curing was applied.
Results and Discussion
Different spectra can be generated by subtracting a spectrum of the uncured glue (defined as a negative-time spectrum) from each of the subsequent positive-time spectra. Figure 2 shows the resulting difference spectra, which highlight the changes produced by the curing reaction in 25 ms increments. Negative bands show that a feature has disappeared, while positive bands show that a new feature has grown in. Here it is evident that not one, but two new bands grow in, at ca. 1638 and 1624 cm-1.
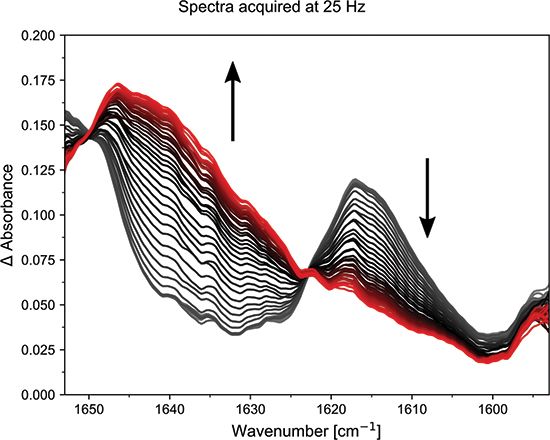
Figure 2: Time evolution of the curing reaction acquired in 25 ms time increments.
The fringing, expected when using a laser-based instrument with an ATR prism, is caused by a change in the refractive index of the sample (for example, glue vs. air). Fringes could be removed with a simple Fourier transform (FT) filter.
The kinetic traces at two points in the spectra (inset) clearly show the time-point where the lamp was turned on. By fitting the growth and decay of these points, the kinetic properties of the curing process can be determined.
Conclusion
Rapid measurements with high signal-to-noise ratios were performed on a sample of glue during curing, giving insight into processes that happen on a millisecond timescale. This bridges the previously demonstrated capabilities of the IRis-F1 to measure on the microsecond timescale, with the second-to-minute timescale. It enables processes over many orders of magnitude in time to be studied.
IRsweep AG
Laubisruetistrasse 44, 8712 Staefa, Switzerland
tel. +41 (0)44 545 85 99
Website: www.irsweep.com
