Trace Element Analysis of Heavy Metals in Pharmaceutical Materials
One of the most common product safety-related analytical tests in the pharmaceutical industry (often referred to as a Limit Test) is the quantification of heavy metals or inorganics in all materials within a pharmaceutical product.
Ian Campbell, PANalytical B.V.
One of the most common product safety-related analytical tests in the pharmaceutical industry (often referred to as a Limit Test) is the quantification of heavy metals or inorganics in all materials within a pharmaceutical product. This normally includes the toxic heavy metals such as As, Hg, and Pb. It is also common practice to measure catalysts, such as Pd and Pt, which are also well classified toxic elements. Throughout the manufacturing processes there are many potential sources of contamination. Therefore, in addition to measuring the starting materials it is essential to measure all finished products and in some cases intermediates, to demonstrate the compliancy with the various regulations.
The MiniPal 4 Pharma is an EDXRF benchtop instrument that is designed for the analysis of a wide variety of elements from Na to U, in concentrations from 100% down to μg/g levels. To demonstrate the performance of the MiniPal 4 Pharma, seven heavy metals representing contaminants, stainless steel residuals, and catalysts that are commonly analyzed were tested in pharmaceutical materials. This rapid analysis was conducted with minimal sample preparation.
Preparation of standards
A common excipient material (pharmaceutical grade cellulose) was chosen as a base material. In-house standards were prepared using ultrapure commercially available organometallic standards comprising seven heavy metals. Standard concentrations were confirmed by ICP-MS.
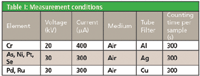
Table I: Measurement conditions
Instrumentation and Measurement Conditions
The measurements were performed using a MiniPal 4 Pharma EDXRF spectrometer, equipped with a molybdenum anode X-ray tube, 5 tube filters, a helium purge facility, and a high-resolution silicon drift detector. Loose powder samples, weighing approximately 2500 mg were placed directly into disposable sample cups with an X-ray transparent polypropylene foil (6 μm). All analyses were performed in an air atmosphere.
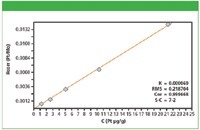
Figure 1: Calibration plot for Pt in cellulose.
The measurement conditions for the various heavy-metals in a cellulose matrix are listed in Table I. Multiple elements can be measured with a single condition set. This degree of simultaneous data collection led to a total counting time of 15 min per sample.
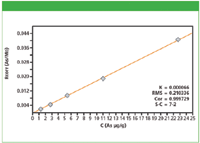
Figure 2: Calibration plot for As in cellulose excipient material.
Calibration Results
Calibration graphs were set up using the regression model of the MiniPal software. Figures 1 and 2 show calibration plots for Pt and As using multiple in-house standards of cellulose doped excipient materials. The plots demonstrate a good correlation between the expected concentration and the measured intensities. The calibration results for all analyzed elements are shown in Table II. The root mean square (RMS) error listed in Table II is a measure of the difference between the calculated concentration and the chemical concentration and is therefore a measure of the accuracy of the method. A weighted error estimate, the K-value, is independent of the concentrations and gives a more reliable indication of accuracy. Lower K-values indicate a more accurate calibration.
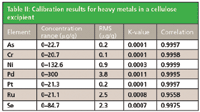
Table II: Calibration results for heavy metals in a cellulose excipient
Analytical Precision
To demonstrate the long-term analytical precision of the MiniPal 4 Pharma spectrometer, one excipient sample was measured 700 times without any recalibration. The average concentration and RMS error are presented in Table III. The precision for Ni and As are illustrated graphically in Figure 3.

Table III: Excipient detection limits using application method settings
Detection Limits
The detection limits (using the application measurement times) for the metals studied are presented in Table III. Here two commonly used excipient materials are compared representing the range of limits that can be expected with most organic pharmaceutical products.
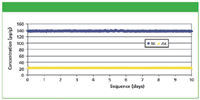
Figure 3: Precision results (approximately 700 measurements over 10 days) for Ni and As in celulose excipient without recalibration.
Conclusion
The results from this study clearly demonstrate that the MiniPal 4 Pharma EDXRF spectrometer is well suited for the analyses of pharmaceutical materials including excipients, API's, and finished products. The repeatability results demonstrate the long-term stability and robustness of the MiniPal 4 Pharma. Good results are demonstrated for the regressions and lower limits of detection. Furthermore, the compact size and low weight of the spectrometer makes it an ideal system for investigations and limit testing of heavy metals in pharmaceuticals.

Figure 4: MiniPal 4 Pharma spectrometer.

PANalytical
117 Flanders Road, Westborough, MA 01581
tel. (508) 647-1100, (800) 892-7174
Website: www.panalytical.com
