Photoacoustic Spectroscopy
September 2006. An unusual form of spectroscopy uses light and sound to probe the behavior of materials. Here is a brief introduction to photoacoustic spectroscopy.

There is a form of spectroscopy known as photoacoustic spectroscopy. As with many forms of spectroscopy, its name is descriptive. The prefix "photo" makes sense for a form of spectroscopy, but "acoustic"? Doesn't that mean "relating to sound"? Is there really a form of spectroscopy that uses sound?

David W. Ball
Yes, there is.
In 1880–1881, Alexander Graham Bell (1–3) found that when a thin disk was exposed to mechanically chopped sunlight, sound was emitted. In addition, he noted a similar effect when infrared or ultraviolet light was used. A plot of the loudness of the sound versus, for example, the wavelength of the light used, is called a photoacoustic spectrum. According to Haisch and Niessner (4), this effect essentially was forgotten until researchers led by Allen Rosencwaig (ironically) at Bell Labs rediscovered the behavior and provided a theoretical basis (5). To indicate the growth of the field, Figure 1 shows the number of publications listed by the Chemical Abstracts Service under the subject "photoacoustic spectroscopy".
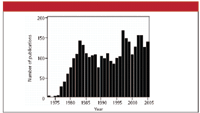
Figure 1: Number of articles published using the key phrase "photoacoustic spectroscopy" since the early 1970s.
Photothermal Techniques
Photoacoustic spectroscopy is part of a class of photothermal techniques, in which an impinging light beam is absorbed and alters the thermal state of the sample. This "thermal state" can manifest itself as a change in temperature, density, or other measurable property of the sample. One method of detection is to experimentally measure the temperature or density of the absorbing material. This is referred to as thermometric detection.
However, if the incoming light is modulated, the absorbing sample warms and cools in a cycle. If the cycle is so fast that the sample does not have time to expand and contract in response to the modulated light, a change in pressure develops. This pressure "wave" can lead to the production of a sound wave. These sound waves can be detected by a sensitive microphone, piezoelectric devices, or optical methods (for example, deflection of highly collimated light reflecting from the surface). These techniques are more properly called photoacoustic techniques. It has been argued that photo-acoustic spectroscopy is as much a type of calorimetry as it is spectroscopy (5).
Photoacoustic Spectroscopy: Gases
One advantage to photoacoustic spectroscopy is that it can be performed on all phases of matter. Figure 2 shows a general setup for the photoacoustic spectroscopy of a gas sample. When a species absorbs some of the incoming light, one of several mechanisms of de-excitation is intermolecular colliding, which ultimately leads to increases in translation energy of the gas particles — that is, heating. According to the various gas laws, an increase in the temperature of the gas leads to an increase in the pressure of an isochoric (constant-volume) sample. If the incoming light is modulated — modulation frequencies can vary from single to several thousand hertz — the gas pressure increases and decreases accordingly, creating sound. Varying the wavelength of the incoming light will change the amount of light absorbed, the amount of pressure changes occurring, and the amount of sound produced, and a spectrum of loudness versus wavelength can be produced.
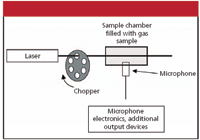
Figure 2: General experimental setup for performing photoacoustic spectroscopy on a gas.
One useful aspect of photoacoustic spectroscopy of gases is that very bright light sources — lasers — can be used to detect very tiny concentrations of a particular gas, on the order of parts per trillion. This makes photoacoustic spectroscopy very useful in following the concentrations of trace gases in mixtures, like soot in diesel exhaust or NOx in the atmosphere. Photoacoustic spectroscopy can also be limited because laser light is not very broad in bandwidth; the analyte molecule must absorb some light from the source in order to be detectable. Saturation effects can also cause problems (6).
Photoacoustic Spectroscopy: Condensed Phases
The mechanism of the photoacoustic effect in condensed samples is not as straightforward as it is for gas samples. For example, in 1973, Parker (7) noticed a photoacoustic signal apparently coming from the windows of the sample cell, which should have been transparent to the incoming radiation. Further (and this might be unique to this particular form of spectroscopy), the exact mechanism of spectrum production depends on the type of detector used. The commonly accepted mechanism for the photoacoustic effect is called RG theory, after its developers Rosencwaig and Gersho (8,9). The main source of the acoustic wave is the repetitive heat flow from the absorbing condensed-phase sample to the surrounding gas, followed by propagation of the acoustic wave through the gas column to microphone-based detector.
However, a photoacoustic signal also can be detected piezoelectrically. Instead of being dissipated as heat, the absorbed radiant energy also can be transferred through the solid-state vibrational modes, or phonon modes, of the sample. The motions of these phonon modes are nondissipative (unlike heating), limited only by the size of the sample. A piezoelectric detector physically connected to the sample can detect absorbed energy in this manner. Although piezoelectric detection is about 100 times less sensitive than microphone detectors, it can be preferable for large samples or for samples that do not efficiently convert absorbed light to heat.
Other Issues
One advantage of photoacoustic spectroscopy is that it is nondestructive to the sample; the sample does not have to be dissolved in some solvent or embedded in a solid-state matrix. Samples can be used "as is." Another advantage is the potential for performing depth profiles of analytes in optically transparent media. Figure 3 shows how this is possible: essentially, the slower the modulation rate, the farther the heat can diffuse before the incoming light is cut off. Figure 4 shows examples of infrared photoacoustic spectra of poly(vinylidene chloride) films using different modulations, showing differences in the spectra due to differences in penetration depth.
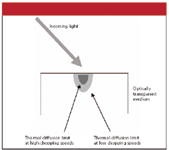
Figure 3: How photoacoustic spectroscopy can perform depth profiling.
Photoacoustic spectroscopy is not only used for depth profiling. As indicated earlier, it can be used to measure pollutants such as NOx or soot in the atmosphere or in automobile exhaust. Haisch and Niessner (4) also list the analysis of textile dyes as another practical application of photoacoustic spectroscopy. Photoacoustic spectroscopy is particularly useful for samples that are powdered (like catalysts), amorphous, or otherwise not conducive to reflective or transmission forms of optical spectroscopy. The photoacoustic effect is used to study biological samples such as blood, skin, eye lenses, tumors, and drug-laced tissues. Several studies are available in which photoacoustic spectroscopy has been used to identify different types of bacteria. Interested readers are urged to consult the literature to see if this fascinating form of spectroscopy might be right for them.
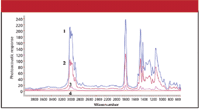
Figure 4: Fourier transformâinfrared photoacoustic spectra of poly(vinylidene chloride) films at four modulations. Spectrum 1: 50 Hz; spectrum 2: 100 Hz; spectrum 3: 400 Hz; spectrum 4: 1000 Hz. (Reprinted with permission from reference 10. Copyright 2004 American Chemical Society.)
David W. Ball is a professor of chemistry at Cleveland State University in Ohio. Many of his "Baseline" columns have been reprinted in book form by SPIE Press as The Basics of Spectroscopy, available through the SPIE Web Bookstore at www.spie.org. His most recent book, Field Guide to Spectroscopy (published in May 2006), is available from SPIE Press. He can be reached at d.ball@csuohio.edu his website is academic.csuohio.edu/ball.
References
(1) A.G. Bell, Am. J. Sci. 20, 305 (1880).
(2) A.G. Bell, Phil. Mag. 11, 510 (1881).
(3) An article describing the work in the journal The Manufacturer and Builder 13(7), 156–158 (1881) is available online through Cornell University at http://cdl.library.cornell.edu/cgi-bin/moa/moa-cgi?notisid=ABS1821-0013-416.
(4) C. Haisch and R. Niessner, Spectroscopy Eur. 14(5), 10 (2002).
(5) A. Rosencwaig, Photoacoustics and Photoacoustic Spectroscopy (R.E. Krieger Publishing Company, Malabar, Florida, 1980).
(6) A. Rosencwaig, Photoacoustics and Photoacoustic Spectroscopy (R.E. Krieger Publishing Company, Malabar, Florida, 1980), p. 69.
(7) J.G. Parker, Appl. Opt. 12, 2974 (1973).
(8) A. Rosencwaig and A. Gersho, Science 190, 556 (1975).
(9) A. Rosencwaig and A. Gersho, J. Appl. Phys. 47, 64 (1976).
(10) P. Zhang and M.W. Urban, Langmuir 20, 10691–10699 (2004).

Best of the Week: Microplastic Pollution, Previewing the AAFS Conference, Next-Gen IR Sensors
February 21st 2025Top articles published this week include an interview that provides insight into how marine monitoring can improve mitigation of plastic pollution, coverage of the American Academy of Forensic Sciences (AAFS) conference, and an article about next-generation infrared (IR) sensors.
Can Fluorescence Spectroscopy Evaluate Soil Dissolved Organic Matter Dynamics?
February 20th 2025A new study published in Chemical Engineering Journal by researchers from Northeast Agricultural University in China reveals that biochar aging, influenced by environmental factors like UV exposure and wet-dry cycles, alters dissolved organic matter composition and affects its effectiveness in remediating cadmium-contaminated soil.
Next-Generation Infrared Sensors: Innovations in Semiconductor Materials and Applications
February 19th 2025A recent study provides an in-depth overview of the latest advancements in infrared (IR) semiconductor sensor technology, highlighting new materials, enhanced detection capabilities, and expanding applications across industrial, medical, security, and environmental fields. The research explores how quantum dots, graphene, and novel nanomaterials are revolutionizing IR detection, paving the way for more efficient and versatile sensor systems.