Are You Getting Value from Your Spectrometer?
Getting the best business value from a spectrometer requires knowledge of the instrument and its operating abilities, any attachments, the sample including sampling procedure and presentation, and the software. All of these elements must be pulled together by a skilled and knowledgeable spectroscopist. Unfortunately, this is not always the case in many organizations.

In a dark, dark cupboard far, far away from the boss's prying eyes, somewhere in your laboratory, is usually that spectrometer you wish you'd never bought. It was purchased at phenomenal expense and with great promise but it did not match reality when used operationally. So over time, alternative analytical procedures have been developed or you stick with the existing ones that the new instrument was meant to replace and you push the spectrometer into the cupboard and hope nobody notices. We've all done this at some time in our careers, mainly because we did not specify our requirements in sufficient detail or understand exactly why we were purchasing the system. Provided that we learn from the experience and don't repeat it, we know that we should get better at instrument selection by documenting our user needs in the first place. Hiding an instrument from view keeps the management heat from you but does not assuage your conscience. This is the most obvious case of poor instrument selection.

Robert D. McDowall
However, the purpose of this "Focus on Quality" installment is to look at what happens after you have purchased a system and determine the factors you need to get right to enable the system to deliver what was promised. This is especially so for molecular spectrometers such as infrared, near infrared (NIR), and Raman instruments, which offer the potential for great business benefit but often can fail to deliver. During laboratory audits that I have conducted in the past year, I have seen examples where spectrometers have failed to meet their full expectation and potential. Therefore, I want to discuss with you some of the main points that I think need to be considered to get the most out of your spectrometers and save resources at the same time.
The Promise: Taking the Laboratory to the Sample
Molecular spectroscopy (for example, IR, NIR, and Raman) is an analytical technique that promises rapid identity verification or qualitative analysis of most chemicals by nondestructive testing, often in situ. Furthermore, it also is capable of quantitative analysis using chemometric models contained in the instrument software, provided that the system has been correctly set up. In both modes, it is quicker than the alternative of destructive testing that occurs in conventional quality control laboratories where production needs to wait while the laboratory checks if the material meets the appropriate specification.
The basis of a spectrometric verification test, often carried out in a warehouse, is that the supplier of the material has tested and certified the material against your specification and all you are doing is identity confirmation. However, there is the ability to use spectroscopic analysis to differentiate chemicals with the same structure but different physical properties — for example, differentiating different particle sizes of microcrystalline cellulose. However, to be successful, a library of spectra needs to be built up and where necessary, the postspectrum analysis models need to be established as part of the overall analytical procedure and then the overall analytical procedure must be validated.
If the procedure is set up correctly, it can save a company a lot of money and time and avoid errors. For example, if a system is installed in a goods-inwards warehouse, as the containers of material come off the delivery truck, they can be sampled and tested almost immediately. If the identity is wrong, the containers can be loaded back on the track and returned to the supplier. The company has not paid for the shipment and the material has not been taken through a long and expensive QC testing process before the error has been found. Thus, there is an immediate benefit on company cash flow. Furthermore, it can enable a just-in-time approach to manufacturing and hold less stock, further improving its cash position. As it can differentiate between polymorphs of the same compound, it also can be used to detect counterfeit products or subtle changes in the manufacturing process of the compound.
When these spectroscopic techniques are used for quantitative analysis, there is the possibility to determine the purity of a material, either as a raw material or a finished product as well as large levels of contaminants or impurities (down to ~1%). Also, many users of spectrometers don't realize the wider benefits of the technique as the instrument gathers much data that are unused: for example, moisture can be determined simultaneously and time saved by avoiding a separate Karl Fischer test. With the rise of counterfeit goods, molecular spectroscopy is being used both qualitatively and quantitatively to analyze suspected goods and compare with the genuine article. The ability to achieve analyses such as these requires that the system is set up correctly. However, to reach this spectroscopic nirvana, the laboratory must overcome some obstacles that might not always be considered and hence, the predestined journey to that dark, dark cupboard.
But Often Reality Does Not Match the Promise
So the theory and promise are great, but what happens in reality? The workflow goes something like this after the selection and purchase of the instrument (any similarity to your laboratory is merely coincidental):
- You will have written down your requirements for the system and have a good understanding of what analyses it will be undertaking when operational. You'll have compared the instruments available on the market with your specification and made the selection based upon fit to requirements, ease of use, price versus budget, and support by the potential vendor. Why are pigs flying around my office as I write this section?
- The selected system is delivered by the vendor to your laboratory.
- In a minority of cases, mainly regulated laboratories, the system will sit in the boxes for three to six months as the spectroscopists do not have time to validate the system. In the majority of cases, we will move immediately onto the next phase of the process.
- The vendor's service engineer will unpack the system, place it in the location where it will be used, install the components including the software and any sampling attachments purchased, and then integrate them into a system.
- The service engineer will then test the system to be confident that it works from the vendor's perspective. There will be varying degrees of formality at this stage depending if the laboratory is regulated or not and how much the company has paid for this part of the service.
- Finally, the service engineer will hand the system over to the laboratory as now it is working from the vendor's perspective.
This part of the workflow is shown in the top half of Figure 1. This is the easy bit and you are now the proud owner of a brand spanking new spectrometer that has been commissioned by the vendor! So all you have established at this stage of the process is that the instrument and the other system components meet the vendor's specifications. However, this does not mean that your analytical procedures or the software analysis models used in them have been established or validated, nor does it mean they are usable.

Figure 1
So what are you going to do now? Establish or transfer and validate existing analytical procedures and methods using the new system. Alternatively, you will need to develop and validate new ones. You will probably need to configure the software to do what you want and spend time building the spectroscopic library if you want to perform identity tests. Furthermore, you may need to develop the chemometric models if you need to interpret the spectra acquired from sample analysis if performing quantitative analysis. Of course, this work has all been planned in advance and you have allowed time for all of these activities? Here's where the fun starts. You are now at a crossroads with two paths ahead of you, one to the left and the other to the right. Where are we going? The signpost for the right-hand path reads "Heaven" and for the left-hand path it says "Hell." Reading the guidebook, we find that Heaven is the spectroscopic nirvana where the system provides business benefits and is used well. Conversely, Hell closely resembles the dark, dark cupboard in your laboratory that you use to hide your mistakes. Are we going to turn left or right?
There are a number of factors that could influence which path you will eventually take. Listed below are some of the major issues to consider along with a brief description of each. How many of these will apply to your laboratory will depend upon how you will use the system.
- Defined purpose for the spectrometer: You will have done this by writing down your requirements used to select the instrument. However, have you captured all of the ways you will use the instrument or just the main one? Just documenting the user needs does not ensure that the system will deliver it. So you will need to know the operational limits of the instrument — will you be working close to any limits?
- Analytical technique and procedure: The procedure is documented and has been validated is a given to ensure that the results are scientifically sound. However, how are you using the instrument? Is the method qualitative or quantitative? Does it use transmittance or reflectance mode?
- Sampling and the container that will be used will be either established or must be investigated as well as the way the sample is presented to the instrument.
- The use of any attachments used in an analysis,such as an autosampler or a fiber-optic probe, should be understood
- Software, including the development of any chemometric models used to analyze or interpret acquired spectra or reference library generation and maintenance, will be known and understood by the developer
Unfortunately, as you failed to plan for this work, you have just turned onto the left-hand path. Furthermore, you have just been joined on your journey by the devil's disciple: laboratory management! The common management mantra is get the instrument working, and now (I could say yesterday but do not want to spoil your day so early into this column). The rational is understandable, as there has been a large investment in purchasing the system and a return on investment is required for senior management. However, the old maxim "failing to plan is planning to fail" rears its ugly head at this point, and the spectrometer is on the downward spiral to that dark, dark cupboard.
What Do We Need to Achieve the Promise?
The first thing to remember is that for many applications, spectrometers do not simply come with on buttons. There is more to using the instrument and system effectively than that. The purchase, installation, and vendor commissioning phases of the work are relatively straightforward as we can see from Figure 1. The problems come when a laboratory wants to use the system for a specific analytical procedure that requires the spectrometer and the software to do more than just print a spectral scan. We will consider just a few of the factors that will influence the path you will take with your spectrometer.
Of the critical factors needed to reach the promise offered by spectroscopy, we will discuss the following ones:
- Instrument capability: What can the instrument can do versus the job you want it to do? Are the two congruent?
- Software skills: Understanding what the software can do and its limitations for your specific analytical applications and chemometric models.
- Accessories: Do you know how to use them and what impact they have on the resulting spectra?
- Sample presentation: Putting the sample in a suitable container before analysis is always critical to the outcome of any analytical procedure and then presenting the sample to the instrument.
If we return to the situation in which a spectrometer is to be used to verify the identity of chemicals entering a warehouse, we will need to generate a spectral library that will use the instrument and the software. The amount of time that you will need will depend upon the amount of discrimination you require. For example, if you want to discriminate lactose from methylene chloride, you will only require one or two sample spectra of each compound. For materials that are more closely related then, you will need to use up to 30 batches from the same supplier who used the same manufacturing process for each chemical. The reason for this is that differences in the manufacturing process over time will result in small changes in spectra. To ensure good discriminatory power of the instrument, the library must have sufficient examples. You can build a library with fewer samples but you will lose discrimination. If you decide to go down this route, consider what could happen if a mistake were made and the wrong material accepted.
Taking the library-development discussion further, if very closely related material must be discriminated, then chemometric models, using partial least squares (PLS), principal components regression (PCR), or soft independent modeling of class analogy (SIMCA) analysis as examples, must be developed and validated. These will take correspondingly more time, and the complex ones can require 3–9 months before they are fully developed, validated, and operational. The problem is that management needs to be aware of the time needed to do this and plan accordingly.
Therefore, for qualitative analysis, the message is: You need to plan for some or all of the following questions:
- What you will use the library to identify?
- How many compounds will be entered in the library?
- How closely related chemically are the compounds?
- How many batches do you need for each chemical?
- Do you need to consider chemometric models?
So far, we have just looked at factors influencing the library. Another factor in the mix will be if sample accessories for the spectrometer are used in an analytical procedure:
- Autosampler for vials, powders, or tablets
- Liquid sipper
- Diffuse reflectance module
- Remote fiber-optic probes for either solids or liquids
Accessory choice can be critical, and when each is used in an analytical procedure, it can influence the outcome. Therefore, accessory choice needs to be understood. The choice of accessory or accessories will impart their own effects on the spectra obtained by the instrument and therefore, the laboratory must know which ones will be used at the beginning of method development so that the library or model can be built using them from the start and avoid repeating work unnecessarily.
Consider also the method: How robust or accurate does the procedure have to be as the users of the procedure might not be spectroscopists or even analytical scientists? For example, there is always a balance between accuracy and robustness of the method. There can be many reasons for this but one is based upon the beam size of the instrument used in the procedure, which illustrates the need to know and understand this relationship. If you want a robust assay then typically a larger beam size is used, but this is at the cost of the accuracy of the result. Conversely if you want accuracy a smaller beam size can be used but at the cost of method robustness. In turn, this decision can result in more work on developing a library of the model used in the assay. Does this matter? Only you can decide based upon your analytical needs but the point I am making is that you need to understand how the instrument and software operate so that you can make an informed decision: Know the performance characteristics and operating envelope of the instrument you are using.
How the sample is presented to the instrument is also important and is shown in Figure 2. The standard sample jars used for QC analysis were suggested for use with a new NIR spectrometer to be used for sample identification in a warehouse. When sample jars were checked, there was a high background caused by the curvature of the bottom of the jar, which dispersed the light beam. This is shown as the blue line in Figure 2. A 22-mm vial was better suited to the analysis as the base of the container was flat and the glass thinner than the standard jar, resulting in lower absorbance losses. As can be seen in Figure 2, with the red line, the resulting spectrum has a lower background and shows more detail than using the standard jar. Interestingly, which container did the laboratory select for their sample container, the jar or the vial? Yes, you guessed right, it was the jar. Not for scientific reasons, but mainly for cost, as the vials were more expensive than the vials and they did not wish to modify existing working practices. Furthermore, the compounds in the spectral library were relatively divergent and therefore, the spectral detail obtained from using the vial was not needed. However, if the spectral library is increased and closely related compounds needed to be identified, this changes the whole approach. The downside of the decision is that the vial probably will be needed for high-resolution work and the library would need to be completely regenerated and validated. The message of the sample container saga is that you must think through your use of the spectrometer carefully and to plan ahead as much as possible.

Figure 2
This is not an all-inclusive list of factors that can influence the use of a spectrometer. Some of the others are
- Interoperability between instruments from the same vendor
- Interoperability between instruments from different vendors
- Interchangeable custom spectral libraries
- Library maintenance over time
- Use of third-party software
Key to a Successful Solution Is an Expert User
We have discussed a number of technical and scientific issues surrounding the effective deployment of a spectrometer but we have omitted the one key factor that will ensure success by integrating all of these factors together: an expert user. The decision of this debate is who is going to drive the instrument? This, in my view, will determine if you are going to be successful or if the dark cupboard will loom large in your thoughts.
To understand how a spectrometer can be used effectively, it is important to understand the continuum of users of a system, and this is shown simply in Figure 3. We have on the left-hand side of the continuum a trained user or operator who would follow a set of instructions to perform a method but perhaps without understanding the technical issues of the instrument. On the right-hand side we have the expert user who understands the technical issues and can develop and validate methods for the trained users to perform. These are the extremes of the continuum. In the middle of the continuum there may be users who have more technical understanding of the system and others who are analytical scientists but have had little training in the instrument other than that offered by the service engineer augmented by reading the manuals. So if you want to develop complex spectroscopic methods, who will you be calling (as a clue it will not be Ghostbusters)? That's right, the expert user! It was the lack of these individuals that caused me to write this column in the first place when I was auditing two laboratories.

Figure 3
Let us look in a little more detail at the skills this group should have for effective development of spectroscopic methods. This is shown diagrammatically in Figure 4.
The issue in a climate of strict cost control: Do you invest in the skills of an expert spectroscopist who understands the theory of the technique, knows the instrument and its capabilities, and how to use the accessories effectively, along with the appropriate software skills? Or do you use a generalist user? To get the most from your instrument and software, you will need a spectroscopist with some or all of the skills shown in Figure 4. In the current economic climate, senior management often does not understand this and will cut specialists and aim for generalists, which is a mistake in my view. Another skill that is required by the spectroscopist is management of expectations, as it is clear from reading this column that you will not be able to roll out a complex analytical procedure in 5 min. Keeping management aware of the timeframes required is important.
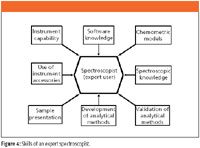
Figure 4
Spectroscopy has great potential to save time and money and the scientists who can deliver this may not be retained by an organization. So if you don't have this in-house, it could be outsourced either from a specialist consultancy or from the instrument vendor. Regardless of the source of skill, a laboratory will need this knowledge and skill if a laboratory is to realize fully the benefits of investing in spectroscopic analysis.
Summary
We have looked at some of the factors necessary for effective and efficient deployment of spectroscopic methods of analysis within an organization. The key element is that an expert spectroscopist is critical for success. How you get this expertise (internal or external available) is up to the organization. However, specialist user expertise is the critical success factor to achieve the promise of spectrometry and get the most value and return on investment from your spectrometer.
R.D. McDowall is principal of McDowall Consulting and director of R.D. McDowall Limited, and "Questions of Quality" column editor for LCGC Europe, Spectroscopy's sister magazine. Address correspondence to him at 73 Murray Avenue, Bromley, Kent, BR1 3DJ, UK.
References
(1) ASTM E 1790–04; Standard Practice for Near Infrared Qualitative Analysis (2004).
(2) ASTM E 2056–04; Standard Practice for Qualifying Spectrometers and Spectrophotometers for Use in Multivariate Analyses, Calibrated Using Surrogate Mixtures (2004).
(3) European Pharmacopeia 5.0, 2005; Section 2.2.40 Near-Infrared Spectrophotometry.
(4) United States Pharmacopeia XXXII, 2009 General Chapter <1119> Near-Infrared Spectrophotometry
(5) Guidelines for the development and validation of Near Infrared (NIR) spectroscopic methods; Pharmaceutical Analytical Sciences Group (PASG) October 2001 (http://www.pasg.org.uk/NIR/NIR.htm).
(6) Note for Guidance on the use of Near Infrared spectroscopy by the pharmaceutical industry and the data required for new submissions and variations; CPMP/QWP/3309/01, February 2003 (www.emea.europa.eu/pdfs/human/qwp/330901en.pdf).
