DNA Adductomic Analysis by Data-Independent Mass Spectrometry
Special Issues
A new type of analysis called “wide-selected ion monitoring (SIM)/MS2” scanning, is capable of screening for a wide range of DNA adducts (chemical modifications to genomic DNA). This method has successfully identified DNA adducts from carcinogen exposures and oxidative stress in human prostate and kidney tissues.
Recent advances in liquid chromatography–mass spectrometry (LC–MS) instrumentation have allowed researchers to tackle new analytical challenges in systems biology and toxicology. Untargeted data-independent acquisition scanning methods have emerged in the fields of proteomics, metabolomics, and lipidomics. DNA adductomics is a developing methodology that can detect DNA adducts (chemical modifications to genomic DNA) of many genotoxicants and reactive oxygen species. The University of Minnesota’s Masonic Cancer Center and Department of Medicinal Chemistry has developed an analysis termed “wide-selected ion monitoring(SIM)/MS2 ” scanning, which is capable of screening for a wide range of DNA adducts. Wide-SIM/MS2 involves sequential, alternating acquisition of high-resolution wide-SIM and corresponding MS2 spectra covering a large mass range. The wide-SIM data analysis approach provides for detection of the parent masses of various compounds (including DNA adducts) present in the sample, whereas the MS2 data allows for detection of the neutral loss of deoxyribose, which identifies a particular compound as a putative DNA adduct. This Wide-SIM/MS2 method has successfully identified DNA adducts from carcinogen exposures and oxidative stress in human prostate and kidney tissues. Wide-SIM/MS2 screening technology can advance our understanding about the role of chemical exposures in DNA damage and disease risk.
Humans are frequently exposed to low levels of hazardous chemicals in the environment, water, their diet, and medicines, and through occupational exposures. Many of these compounds or their reactive metabolites can bind to macromolecules (1). DNA adducts, the covalent modification of DNA by genotoxicants, can serve as measures of chemical exposure and the biologically effective dose. Epidemiological studies have linked various chemical exposures to the increased risk of different cancers in humans. Examples include:
- the exposure to tobacco-specific nitrosamines is linked with lung cancer (2,3)
- occupational exposure to aromatic amines causes bladder cancer (2,4)
- the usage of traditional Chinese herbs containing aristolochic acid is linked to urothelial cancers (5,6)
- the consumption of grains contaminated with the mycotoxin aflatoxin B1 (AFB1) is a risk factor for liver cancer (7,8)
- DNA adducts of some carcinogens induce mutations in critical genes involved in carcinogenesis, such as H-ras and K-ras oncogenes, and the p53 tumor suppressor gene (9–12)
- AFB1 and aristolochic acid are two chemicals for which exposures have been definitively linked to DNA adducts, signature mutational spectra in cancer related genes, and the development of cancer in humans (8,13)
The bioactivation and DNA adduct formation of aristolochic acid and the aromatic amine 4-aminobiphenyl, a bladder carcinogen present in tobacco smoke, are shown in Figure 1. If not repaired, these DNA adducts can induce mutations during cell division (14). DNA adducts of carcinogens are formed at different ring atoms, exocyclic nitrogen and oxygen atoms of the nucleobases, and the phosphate backbone (15). The types of DNA adducts formed are dependent on the structures and chemical properties of the reactive intermediates, and also on the ability of the compounds to intercalate with DNA, which may direct adduct formation to specific nucleophilic sites of the DNA bases (15,16).
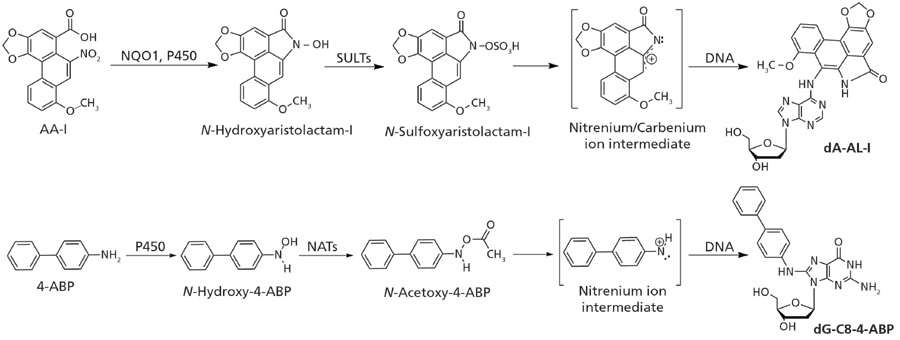
Figure 1: Aristolochic acid-I (AA-I) and 4-aminobiphenyl (4-ABP) are shown as prototypical procarcinogens. AA-I undergoes nitro-reduction through NAD(P)H:quinone oxidoreductase (NQO1), or cytochrome P450. The resulting N-hydroxyaristolactam-I is bioactivated by sulfotransferases (SULTs) to form an unstable N-sulfoxy ester, which quickly undergoes heterolytic cleavage to produce the reactive nitrenium/carbenium ion intermediate that forms dA-AL-I and other DNA adducts. 4-ABP undergoes N-hydroxylation by P450s, then it is further bioactivated by N-acetyltransferases (NATs) to form the N-acetoxy ester, which leads to the formation of dG-C8-4-ABP through the nitrenium ion intermediate.
Methods of DNA Adduct Detection and Challenges
Chemical agents in tobacco smoke, diet, and environmental pollution contribute to damage of the genome. Therefore, chemical analyses of DNA adducts can provide clues about chemicals that contribute to cancer etiology. However, the measurement of DNA adducts in humans is a challenging analytical task, because the levels of DNA adducts usually range between 100 ppb (one adduct per 107 nucleotides) to 1 ppb (one adduct per 109 nucleotides) or less. Moreover, the amount of fresh human tissue is often limited to milligram quantities, and as a result, only low microgram quantities of DNA are available for chemical analyses. Thus, highly sensitive methods are required to screen for DNA adducts. During the past three decades, DNA adducts have been most commonly measured by 32P-postlabeling (17–19), immunoassay–immunohistochemistry (IHC) (20,21), or mass spectrometry (MS)-based methodologies (15,16). MS-based technologies are the most robust and specific methods for screening of DNA adducts, and MS is the only technique that provides physicochemical data to confirm structure identity. MS methods are also quantitative, when employing the stable isotope dilution method (16). Gas chromatography (GC)–MS has been successfully used to measure free radical–mediated oxidative damage of the deoxyribose moiety and the nucleobases, as well as to measure chemically adducted nucleobases and DNA–protein cross-links (22). However, DNA adducts require chemical derivatization to increase their volatility for GC analysis, and the efficiency of chemical derivatization of adducts is a potential issue for robust measurements. Moreover, the DNA adducts must be stable at the elevated temperatures employed in GC–MS. The online coupling of liquid chromatography (LC) or capillary electrophoresis to MS is the preferred method for the measurement of many DNA adducts, particularly those that are thermally labile. LC with electrospray ionization (ESI)-MS is the predominant platform for DNA adduct analysis (15,16,23–26). The rapid advances in DNA adduct measurements have been driven by the recent developments of LC–MS instrumentation and technologies. The high resolving power and accurate mass measurements possible with quadrupole time-of-flight (Q-TOF) and orbital trap MS detectors, as compared to triple-quadrupole and ion trap (IT) instruments, have significantly reduced background noise and enhanced the selectivity of the analyte detection, and the coupling of nano-flow ultrahigh-pressure liquid chromatography (UHPLC) with a nanoESI source has greatly improved the sensitivity. The detection limit of 10-(2'-deoxyguanosin-N2-yl)-7,8,9-trihydroxy-7,8,9,10-tetrahydrobenzo[a]pyrene, a DNA adduct of benzo[a]pyrene, a carcinogen present in tobacco smoke and barbequed meats (2), approaches one adduct per 1011 nucleotides (1 adduct per 10 human lung cells) in the lung of smokers (27).
The analysis of DNA adducts by LC–ESI-MS requires hydrolysis of DNA to modified nucleobases by treatment with acid, base, or elevated temperature, or to modified 2'-deoxyribonucleosides using a cocktail of nucleases. The full-scan high-resolution accurate mass (HRAM) screening of the precursor ions of DNA adducts following acid hydrolysis of DNA has been reported (28); however, tandem MS greatly improves selectivity and sensitivity. The scheme for monitoring DNA adducts at the MS2 scan stage is shown in Figure 2. The most common MS/MS transition feature of DNA adducts is the loss of 2'-deoxyribose (dR, 116 Da, or 116.0473 Da in HRAM) when 2'-deoxynucleosides are subjected to collision-induced dissociation (CID) (Figure 2a) (29). Triple-quadrupole (QqQ) MS instrumentation employing selected reaction monitoring (SRM) or multiple reaction monitoring (MRM) has been the predominant MS platform for targeted DNA adduct analysis over the past two decades because of its fast duty cycle, wide dynamic range, good sensitivity, robustness, and reproducibility (23,30). However, IT-MS has gained popularity in DNA adduct analysis because of its capability of performing multistage MS (MSn) scanning (31). IT with MSn scanning is especially suitable for analyzing modified 2'-deoxynucleosides because DNA adducts typically undergo CID to lose the dR moiety, and the aglycone ions are often detected as the single product ion in the MS2 spectra. In addition, product ion mass spectra of the aglycone adducts [AH2]+ acquired at the MS3 scan stage provide rich structural information about the structure of the adduct and corroborate the identity of the lesion (vide infra). Moreover, the MS3 scanning removes background signals observed at the MS2 scan stage and can be used for robust quantitative measurement of DNA adducts (32).
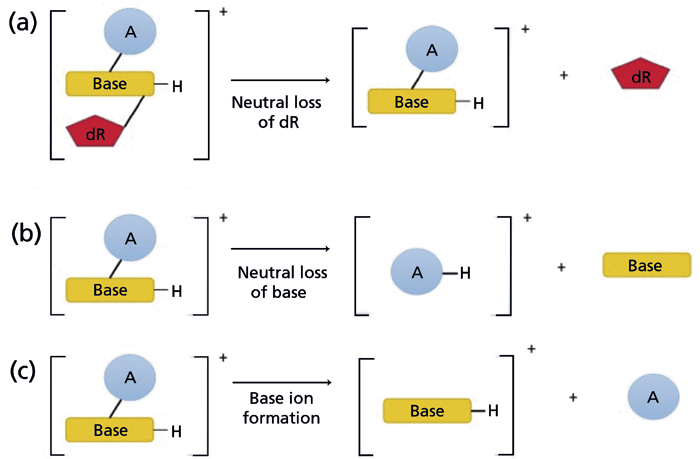
Figure 2: The fragmentation pathways of modified nucleosides analyzed by LC–MS. (a) Most modified nucleosides undergo fragmentation in MS/MS to form the aglycone with the neutral loss of 2'-deoxyribose (dR). Other common fragmentations include (b) the neutral loss of the nucleobase, and (c) the formation of nucleobase ions (34). Copyright MDPI 2017. Adapted with permission from reference 34.
Strategies for DNA Adductomics
Many DNA adduct measurements have been performed by targeting from one to several adducts at a time. However, there are many chemicals in the environment and diet, as well as endogenous electrophiles produced in our bodies, that can react with DNA to form adducts (33). Thus, robust and versatile scanning methods have been sought to detect many DNA lesions in an untargeted fashion in a single run. With the emergence of hybrid orbital trap and Q-TOF MS instruments with flexible scanning events, high resolution, and accurate mass measurements, it is possible to screen for many DNA adducts in a single assay (34). The detection of many DNA adducts at a time, termed DNA adductomics, by different MS approaches has been recently reviewed (34,35). Four principal scanning techniques are currently in use for DNA adductomics (Figure 3):
- a continuous scan of constant neutral loss (CNL) by monitoring mass transitions of adduct precursors ([M + H]+) to their aglycones ([M + H -116]+ in QqQ MS)
- MS2 data-dependent acquisition (DDA) in quadrupole trap (Q-trap), Q-TOF, or orbital trap
- data-dependent CNL of 116.0 or 116.0473 Da that triggers MS3, with or without a targeted mass inclusion list, in Q-trap, linear ion trap (LIT), or hybrid LIT-orbital trap MS
- data-independent acquisition (DIA) in MSE (dynamic switching between low and high collision energy in parallel scans) in Q-TOF, and wide-SIM/MS2 in a hybrid orbital trap MS instrument
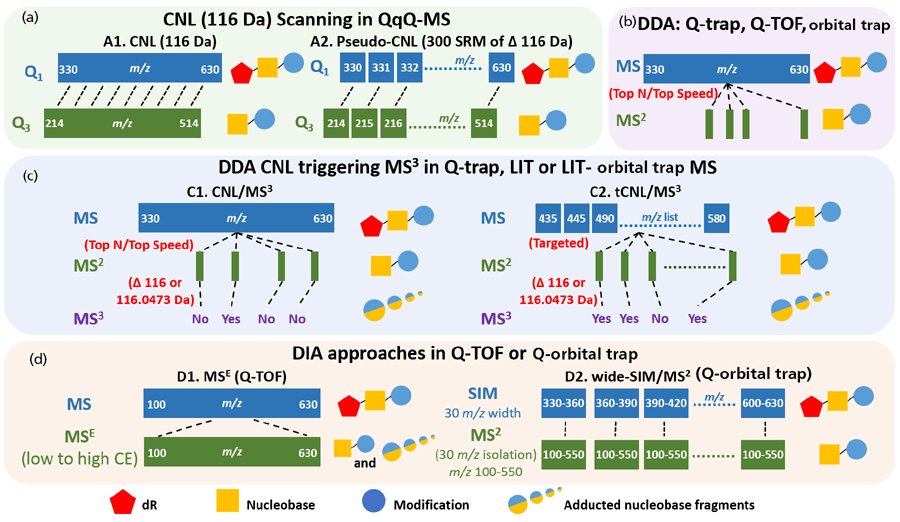
Figure 3: Schemes of DNA adductomic approaches using different mass spectrometric scanning strategies focusing on adducted 2'deoxyribonucleosides with precursors ([M + H]+ ). (a) The continuous scanning of the constant neutral loss (CNL) of 116 Da (dR) in QqQ MS. This approach is achieved either by using the CNL scan mode of 116 Da (left), or in pseudo-CNL by setting up 300 SRM transitions of [M + H]+ → [M – 116 + H]+ (right). (b) The data-dependent acquisition (DDA) of top N (the most abundant N ions) or top speed (acquire as many dependent scans as possible within the cycle time) mode in Q-trap, Q-TOF or orbital trap. (c) The CNL (116 or 116.0473 Da) triggering of MS3 in trap-based MS that can perform multi-stage MS scan (Q-trap, LIT, or LIT-orbital trap). Adduct precursors are fragmented if they are selected by the survey scan in DDA mode (left) or if they are in the targeted inclusion list (right). The subsequent MS3 scan is only triggered if the loss of 116 or 116.0473 Da is detected in the MS2 scan. (d) The adductomics approach using data-independent acquisition (DIA) methods in HRAMS. In Q-TOF (left), a full scan of all precursor ions, including presumed adducts, is acquired followed by MSE (ramping collision energy: low to high). The ions are fragmented to generate the aglycones [M + H – 116]+ and other possible fragment ions. In wide-SIM/MS2 (right) conducted in the Q-orbital trap, all ions, including adduct precursors, are detected in SIM of 30 m/z windows in the orbital trap instrument, and their aglycones are detected in the following MS2 scan.
DDA for DNA Adductomics
DNA adductomics analysis was first achieved by scanning for CNL of 116 Da using fast atom bombardment MS with electric and magnetic sector in 1990 (36). Thereafter, QqQ MS was successfully applied through a "pseudo-CNL" method monitoring over 300 SRM transitions of [M + H]+ → [M + H - 116]+ (37–39), which has superior sensitivity over the traditional CNL scan mode (40). Our laboratory pioneered the data-dependent constant neutral loss scanning followed by triple-stage mass spectrometry (CNL-MS3) in an LIT MS instrument, using the neutral loss of the dR moiety from the protonated adducts ([M + H - 116]+) in the MS2 scan to trigger the acquisition of the product ion spectra of the aglycone ions at MS3 stage (41). Balbo and Villalta extended the method to HRAM MS (42,43). This method employs either "top N" (the most intense N ions) or "top speed" scan mode (acquire with as many dependent scans as possible within the cycle time) with or without specifying an intensity threshold or charge state. A dynamic exclusion function can be applied to restrict the reoccurrence of the same MS2 scans, thus allowing for more putative adducts precursors to be fragmentated. Background ions detected from solvent blank and negative control samples can be added to an exclusion list to improve the detection efficiency of the adducts. In addition, a targeted inclusion list can be constructed to facilitate the detection of a wide range of DNA adducts and maximize the MS sampling and scanning efficiency.
DIA for DNA Adductomics
The DIA approach in "omics" studies is unbiased and data can be mined post-analyses to search for putative DNA adducts. DIA scanning methods typically employ Q-TOF instrumentation and use a wide Q1 window to sequentially isolate all of the precursors, which are subsequently fragmented in the collision cell, and the resulting fragment ions are detected (44). In some cases, a survey scan is also collected, from which precursor ions can be extracted (45,46). The transition for the loss of 2'-deoxyribose [M + H - dR]+ serves as the foundation for many CNL applications in DNA adductomics. The aglycone ions are often the only fragment ions observed in the MS2 spectra acquired by CID with IT MS instruments. In contrast, in other omics analyses, such as proteomics and metabolomics, multiple MS2 fragment ions of peptides (y and b ions) or metabolites are obtained and used as target and qualifier ions for analyte identification and quantification. Thus, in DNA adductomics, the extraction and coelution of precursor and aglycone peaks of DNA adducts are essential feature criteria for the identification of DNA adducts. An MS3 scan provides spectral data on the adducted aglycone to confirm the identity of the adduct against a mass spectral database of DNA adduct standards or for further characterization of structures of novel adducts.
We recently developed the wide-SIM/MS2 methodology to screen for DNA adducts using a hybrid orbital trap MS system (40). The scheme for wide-SIM/MS2 analyses of DNA adducts is shown in Figure 3d (right) and Figure 4. Our method uses online sample enrichment to remove polar components in the enzymatic digestion matrix, such as salts and non-modified 2'-deoxyribonucleosides, which are present at > 1 million-fold higher levels than DNA adducts. In this configuration, wide-SIM/MS2 screens for bulky hydrophobic DNA adducts that are retained on the trapping column. A UHPLC system operating at 300 nL/min flow rate with a 200 mm x 75 µm reversed-phase C18 fused silica column (with a 10-µm orifice emitter) is interfaced to a nanoESI source. The acquisition method contains 10 alternating wide-SIM scans (30 m/z isolation windows across the m/z range 330–630) and high-energy collision-induced dissociation (HCD)-MS2 scans (m/z 100–630), encompassing all precursor ions of DNA adducts under study. The HCD-MS2 approach fragments all precursor ions in the previous wide-SIM scan event. Thus, both precursor ions in wide-SIM and MS2 product ions are detected with the orbital trap MS detector. Potential adducts are identified by extraction and coelution of the precursor ions in the wide-SIM analysis and the aglycones [M + H - 116.0473]+ in the following MS2 scan.
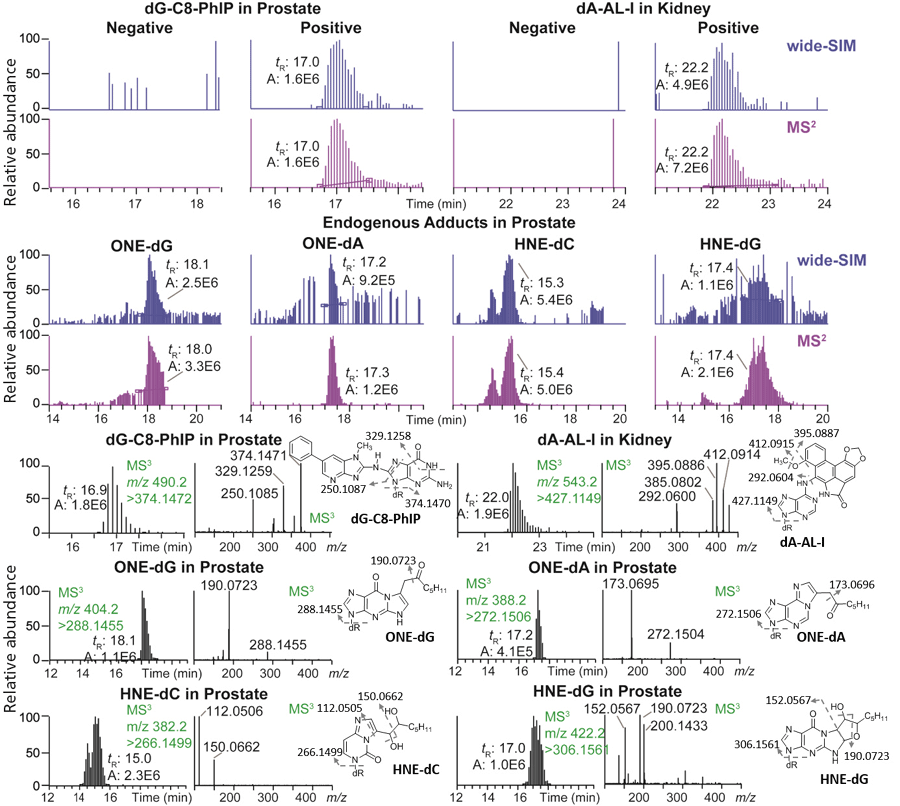
Figure 4: The detection of DNA adducts in human samples by wide-SIM/MS2. dG-C8-PhIP and several endogenous lipid peroxidation adducts were identified in prostate, and dA-AL-I was detected in renal cortex. Adduct structures were confirmed by the MS3 spectra. Precursor and aglycone ions were extracted with a 5 ppm tolerance. The theoretical m/z in wide-SIM and MS2 of each adduct were dG-C8-PhIP, 490.1946 and 374.1472; dA-AL-I, 543.1623 and 427.1149; OHE-dG, 404.1929 and 288.1455; ONE-dA, 388.1979 and 272.1506; HNE-dC, 382.1973 and 266.1499; and HNE-dG, 422.2034 and 306.1561 (40). Copyright ACS 2017. Adapted with permission from reference 40.
The orbital trap MS system utilizes automatic gain control (AGC) to tightly regulate the number of ions or charges entering the orbital trap to avoid space-charging effects that would cause a deterioration in mass resolution and a drop-off in sensitivity (31). Increasing the mass resolution in the orbital trap system can improve the specificity of adduct detection; however, the scan rate is proportionally decreased, and therefore, care must be taken to ensure the resolution settings allow for a sufficient data acquisition rate. Increasing the AGC, within the allowable range, extends the intrascan dynamic range (the maximum abundance ratio between the most abundant and the least abundant signal observable within a given spectrum of ions collected in the trap), thus increasing the likelihood to detect adducts that are present at low abundance (47). However, a large AGC value with increased ion injection times can result in under-sampling and an insufficient number of data points for extraction of the chromatographic peaks. In our analyses, the quadrupole was set to transmit a 30 m/z packet of ions for SIM and MS2, which permitted a satisfactory sampling time and intrascan dynamic range while allowing for a sufficient sampling rate. To take full advantage of in-parallel orbital trap detection and curved linear trap (C-trap) filling for the subsequent mass spectral measurement, the maximum fill time (ion injection time) and the transient length of the specified resolution in the orbital trap must be balanced. For resolving power of 60,000 (full width at half maximum [FWHM] at m/z 200) in the orbital trap MS system, the transient length is about 128 ms. We chose a maximum injection time of 100 ms for AGC limits of 5 × 104 for SIM and 1 × 105 for MS2 to avoid increasing cycle time. Taking into consideration the delay time between scans, the total duty cycle for 20 combined SIM/MS2 scans is about 3 s, which is sufficient to acquire six scans for adduct peaks with an average width of 20 s (FWHM). With these conditions, we showed that DNA adducts of 20 carcinogens derived from chemicals in tobacco smoke, the diet, or lipid peroxidation products spiked in calf thymus DNA (20 µg) before digestion could be identified by wide-SIM/MS2 at levels as low as 0.4 adducts per 108 nucleosides (40). We compared the performance of DIA wide-SIM/MS2 and DDA-CNL-triggering MS3 with a targeted inclusion mass list in the orbital trap instrument, the pseudo-CNL and CNL in QqQ MS using the same samples with identical chromatography and ion source conditions. The overall performance of the four approaches were DIA-wide-SIM/MS2 > DDA-CNL-triggering MS3 with targeted inclusion > pseudo-CNL > CNL, thereby demonstrating that DIA-wide-SIM/MS2 was the most sensitive, robust, and comprehensive method to screen for bulky DNA adducts. However, isobaric interferences can still occur in the SIM detection at 60,000 resolution. The merging of unresolved peaks can shift the observed m/z outside the 5 ppm mass tolerance window and result in the failure to detect the DNA adduct, particularly when they are present at low abundance. For example, the isotopically labeled internal DNA adduct standard of 4-ABP, [13C10]-dG-C8-4-ABP, at the lowest spiking level of 0.4 adduct per 108 nucleotides levels, required a minimum of 200,000 resolution (FWHM at m/z 200) to achieve 10% peak valley separation to detect this adduct at 5 ppm mass tolerance (Figure 5).
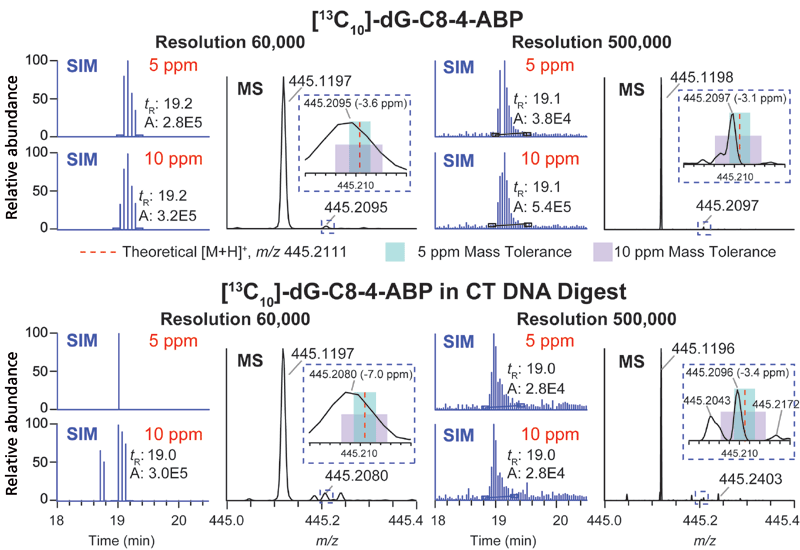
Figure 5: Extracted ion chromatograms (EICs) and mass spectra of precursor [13C10]-dG-C8-4-ABP in pure solvent or in calf thymus DNA digest analyzed by wide-SIM/MS2 at resolutions 60,000 and 500,000 (FWHM at 200 m/z). Precursor ions were extracted at 5 and 10 ppm mass tolerance. The zoomed-in mass spectrum between m/z 445.20 and 445.22 shows the location of the theoretical m/z of precursor ion (445.2111, red dash line), the mass range of 5 ppm (teal box) and 10 ppm (lavender box) (40). Copyright ACS 2017. Adapted with permission from reference 40.
We have compiled a list of over 100 DNA adducts with published MSn fragmentation spectra to facilitate the DNA adductomic analysis by wide-SIM/MS2. The data mining of adducts is performed with LC–MS software by manually extracting theoretical m/z for precursor and aglycone ions at a 5 ppm mass tolerance. The wide-SIM/MS2 scanning technology was used to screen for DNA adducts in human biopsy specimens (Figure 4). A DNA adduct of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), N-(2'-deoxyguanosin-8-yl)-PhIP, was detected in genomic DNA of prostate cancer patients (48). PhIP, formed in cooked meat, is a rodent and possible human prostate carcinogen (49). We also detected a DNA adduct of aristolochic acid, 7-(deoxyadenosin-N6-yl)-aristolactam I (dA-AL-I), in the renal cortex of kidney cancer patients who had ingested herbal medicines (50). Aristolochic acid is a renal carcinogen and a component of Aristolochia herbs used in traditional herbal medicines world-wide (6). A number of lipid peroxidation products were also detected in human prostate and kidney tissues by wide-SIM/MS2 (Figure 4).
Those adducts contained in our data base of DNA adducts with known MS2 fragmentation and neutral losses other than dR (116.0473 Da) can also be mined and broaden the search criteria for different classes of DNA adducts (43). For example, the HCD MS2 analysis of dG-N2-B[a]PDE produced predominant fragment ions of the B[a]PDE moiety, and the protonated aglycone was barely detected (Figure 6). In a similar vein, the combination of CNL and product ion scan employing Q-trap has been used for the untargeted screening of DNA adducts of B[a]P, phenyl glycidyl ether, and styrene-7,8-oxide, where prominent fragment ions in MS2 from the carcinogen moiety were used for adduct identification (51,52). There is only one report using a Q-TOF with MSE to screen for DNA adducts related to oxidative stress in lung of rats exposed to magnetite nanoparticles (53).
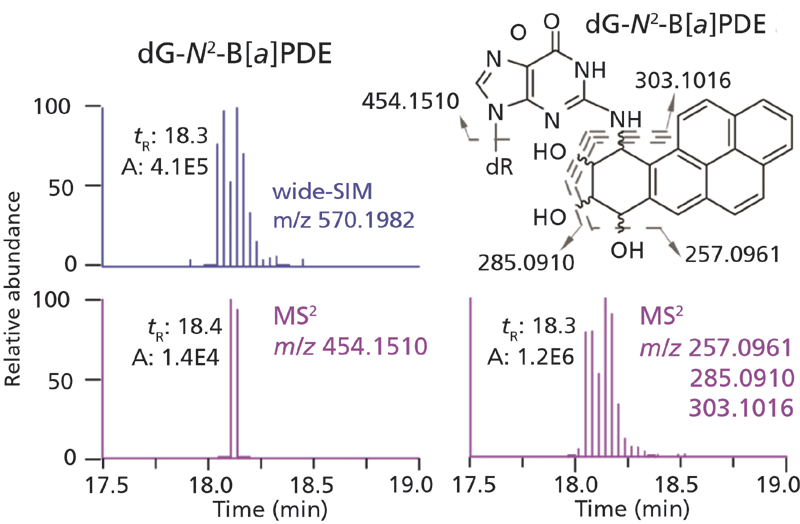
Figure 6: EICs, structure, and fragmentation mechanism of dG-N2 -B[a]PDE analyzed by wide-SIM/MS2. In the MS2 scan, the extracted signal of aglycone is 100-fold lower than that of fragment ions derived from the B[a]PDE moiety (40). Copyright ACS 2017. Adapted with permission from reference 40.
Future Perspectives
The development of bioinformatic tools dedicated for DNA adduct screening and database searching is critical for the advancement of DNA adductomics. Specific to the work presented here, a workflow that includes feature detection of SIM and MS2 chromatographic peaks, alignment of the precursor and aglycone peaks for the identification of the potential known and unknown adducts in an untargeted approach, and the generation of the accompanying mass spectra would allow for the wide-SIM/MS2 methodology to reach its full potential. Also, a larger DNA adduct database is under construction to facilitate automated adduct identification in future applications. Ion mobility spectrometry can be applied to resolve matrix interferences in DNA adductomics (54). Our experiences suggest that further improvements in ion transmission efficiency, the intrascan dynamic range, and more rapid duty cycles will be required for the detection of some DNA adducts present at low abundance. Biopsy specimens are often unavailable for DNA adduct biomarker research. However, formalin fixed paraffin embedded tissues with clinical diagnosis of disease are an accessible, but underutilized biospecimens for screening DNA adducts (55–57). Also, exfoliated cells in saliva (58,59) and urine (60) have been used to screen for DNA adducts through targeted MS approaches, and are promising biospecimens for screening with DNA adductomics. We expect that the advancements of DNA adductomic technologies over the next decade will provide us with a greater understanding of the chemicals that damage the genome and contribute to the development of disease.
Funding
This research was supported by NIH grants R01 CA220367 (R.J.T.), R01 CA122320 (R.J.T.), R01 ES019564 (R.J.T), R50 CA211256 (P.W.V.), and by the Cancer Center Support Grant CA077598.
References
(1) L.A. Stanley, Pharmacognosy, Fundamentals Applications and Strategies (Elsevier Science, Atlanta, GA, 2017), pp. 527–545.
(2) International Agency for Research on Cancer, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 83 (2004).
(3) International Agency for Research on Cancer, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 89 (2007).
(4) International Agency for Research on Cancer, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 38 (1986).
(5) M. Stiborova, V.M. Arlt, and H.H. Schmeiser, Int. J. Mol. Sci. 18, 2144 (2017).
(6) T.A. Rosenquist and A.P. Grollman, DNA Repair 44, 205–211 (2016).
(7) G.N. Wogan, T.W. Kensler, and J.D. Groopman, Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 29, 249–257 (2012).
(8) T.W. Kensler, B.D. Roebuck, G.N. Wogan, and J.D. Groopman, Toxicol. Sci. 120 (Suppl 1), S28–48 (2011).
(9) N.E. Mills, C.L. Fishman, W.N. Rom,N. Dubin, and D.R. Jacobson, Cancer Res. 55, 1444–1447 (1995).
(10) L.A. Peterson, N.M. Thomson, D.L. Crankshaw, E.E. Donaldson, and P.J. Kenney, Cancer Res. 61, 5757–5763 (2001).
(11) T.M. Hernandez-Boussard, and P. Hainaut, Environ. Health Perspect. 106, 385–391 (1998).
(12) M. Hollstein, D. Sidransky, B. Vogelstein, and C.C. Harris, Science 253, 49–53 (1991).
(13) M.L. Hoang, C.H. Chen, V.S. Sidorenko, J. He, K.G. Dickman, B.H. Yun, M. Moriya, N. Niknafs, C. Douville, R. Karchin, R.J. Turesky, Y.S. Pu, B. Vogelstein, N. Papadopoulos, A.P. Grollman, K.W. Kinzler, and T.A. Rosenquist, Sci. Transl. Med.5, 1-10 (2013).
(14) M.R. Stratton, P.J. Campbell, and P.A. Futreal, Nature 458, 719–724 (2009).
(15) S. Liu and Y. Wang, Chem. Soc. Rev. 44, 7829–7854 (2015).
(16) N. Tretyakova, P.W. Villalta, and S. Kotapati, Chem. Rev. 113, 2395–2436 (2013).
(17) K. Randerath, M.V. Reddy, and R.C. Gupta, Proc. Natl. Acad. Sci. USA 78, 6162–6129 (1981).
(18) D.H. Phillips, Mutat. Res. 577, 284–292 (2005).
(19) D.H. Phillips, Cancer Lett. 334, 5–9 (2013).
(20) R.M. Santella, Cancer Epidemiol. Biomarkers Prev. 8, 733–739 (1999).
(21) M.M. Pratt, K. John, A.B. MacLean, S. Afework, D.H. Phillips, and M.C. Poirier, Int. J. Environ. Res. Public Health 8, 2675–2691 (2011).
(22) M. Dizdaroglu, E. Coskun, and P. Jaruga, Free Radic. Res. 49, 525–548 (2015).
(23) R. Singh and P.B. Farmer, Carcinogenesis27, 178–196 (2006).
(24) K. Brown, Methods Mol. Biol. 817, 207–230 (2012).
(25) J.J. Klaene, V.K. Sharma, J. Glick, and P. Vouros, Cancer Lett. 334, 10–19 (2013).
(26) J.M.A. Gavina, C.H. Yao, and Y.L. Feng, Talanta 130, 475–494 (2014).
(27) P.W. Villalta, J.B. Hochalter, and S.S. Hecht, Anal. Chem. 89, 12735–12742 (2017).
(28) L.Y. Hemeryck, C. Rombouts, T. Van Hecke, L. Van Meulebroek, J.V. Bussche, S. De Smet, and L. Vanhaecke, Toxicol. Res.5, 1346–1358 (2016).
(29) S.M. Wolf and P. Vouros, Chem. Res. Toxicol. 7, 82–88 (1994).
(30) N. Tretyakova, M. Goggin, D. Sangaraju, and G. Janis, Chem. Res. Toxicol. 25, 2007–2035 (2012).
(31) J. Guo and R.J. Turesky, Curr. Protoc. Nucleic Acid Chem. 66, 7.24.1–7.24.25 (2016).
(32) A.K. Goodenough, H.A. Schut, and R.J. Turesky, Chem. Res. Toxicol. 20, 263–276 (2007).
(33) C.P. Wild, Cancer Epidemiol. Biomarkers Prev. 14, 1847–1850 (2005).
(34) P.W. Villalta and S. Balbo, Int. J. Mol. Sci. 18, 1870 (2017).
(35) S. Balbo, R.J. Turesky, and P.W. Villalta, Chem. Res. Toxicol. 27, 356–366 (2014).
(36) J. Chaereboudt, E.L. Esmans, E.G. Van den Eeckhout, and M. Claeys, Nucleosides & Nucleotides9, 333–344. (1990).
(37) P.H. Chou, S. Kageyama, S. Matsuda, K. Kanemoto, Y. Sasada, M. Oka, K. Shinmura, H. Mori, K. Kawai, H. Kasai, H. Sugimura, and T. Matsuda, Chem. Res. Toxicol. 23, 1442–1448 (2010).
(38) R.A. Kanaly ,S. Matsui, T. Hanaoka, and T. Matsuda, Mutat. Res. 625, 83–93 (2007).
(39) T. Matsuda, H. Tao, M. Goto, H. Yamada, M. Suzuki, Y. Wu, N. Xiao, Q. He, W. Guo, Z. Cai, N. Kurabe, K. Ishino, Y. Matsushima, K. Shinmura, H. Konno, M. Maekawa, Y. Wang, and H. Sugimura, Carcinogenesis 34, 121–127 (2013).
(40) J. Guo, P.W. Villalta, and R.J. Turesky, Anal. Chem. 89, 11728–11736 (2017).
(41) E.E. Bessette, A.K. Goodenough, S. Langouet, I. Yasa, I.D. Kozekov, S.D. Spivack, and R.J. Turesky, Anal. Chem.81, 809–819 (2009).
(42) S. Balbo, S.S. Hecht, P. Upadhyaya, and P.W. Villalta, Anal. Chem. 86, 1744–1752 (2014).
(43) A. Stornetta, P.W. Villalta, S.S. Hecht, S.J. Sturla, and S. Balbo, Anal. Chem. 87, 11706–11713 (2015).
(44) G. Hopfgartner, D. Tonoli, and E. Varesio, Anal. Bioanal. Chem. 402, 2587–2596 (2012).
(45) R. Bonner and G. Hopfgartner, Bioanalysis 8, 1735–1750 (2016).
(46) A.T. Roemmelt, A.E. Steuer, M. Poetzsch, and T. Kraemer, Anal. Chem. 86, 11742–11749 (2014).
(47) A. Kaufmann and S. Walker, Rapid Commun. Mass Spectrom. 31, 1915–1926 (2017).
(48) S. Xiao, J. Guo, B.H. Yun, P.W. Villalta, S. Krishna, R. Tejpaul, P. Murugan, C.J. Weight, and R.J. Turesky, Anal. Chem. 88, 12508–12515 (2016).
(49) J.S. Felton, I.M. Jagerstad, M.G. Knize, K. Skog, and K. Wakabayashi, In Food Borne Carcinogens; Heterocylclic Amines (John Wiley & Sons Ltd. Chichester, England, 2000).
(50) B.H. Yun, T.A. Rosenquist, V. Sidorenko, C.R. Iden, C.H. Chen, Y.S. Pu, R. Bonala, F. Johnson, K.G. Dickman, A.P. Grollman, and R.J. Turesky, Chem. Res. Toxicol. 25, 1119–1131 (2012).
(51) C. Yao and Y.L. Feng, Talanta 159, 93–102 (2016).
(52) C. Yao, W.G. Foster, J.C. Sadeu, S. Siddique, J. Zhu, and Y.L. Feng, Sci. Total Environ. 575, 742–749 (2017).
(53) K. Ishino, T. Kato, M. Kato, T. Shibata, M. Watanabe, K. Wakabayashi, H. Nakagama, and Y. Totsuka, Int. J. Mol. Sci. 16, 3474–3492 (2015).
(54) A. Kafle, J. Klaene, A.B. Hall, J. Glick, S.L. Coy, and P. Vouros, Rapid Commun. Mass Spectrom. 27, 1473–1480 (2013).
(55) B.H. Yun, T.A. Rosenquist, J. Nikolic, D. Dragicevic, K. Tomic, B. Jelakovic, K.G. Dickman, A.P. Grollman, and R.J. Turesky, Anal. Chem. 85, 4251–4258 (2013).
(56) J. Guo, B.H. Yun, P. Upadhyaya, L. Yao, S. Krishnamachari, T.A. Rosenquist, A.P. Grollman, and R.J. Turesky, Anal. Chem. 88, 4780–4787 (2016).
(57) B.H. Yun, S. Xiao, L.H. Yao, S. Krishnamachari, T.A. Rosenquist, K.G. Dickman, A.P. Grollman, P. Murugan, C.J. Weigh, and R.J. Turesky, Chem. Res. Toxicol. 30, 2130–2139 (2017).
(58) E.E. Bessette, S.D. Spivack, A.K. Goodenough, T. Wang, S. Pinto, F.F. Kadlubar, and R.J. Turesky, Chem. Res. Toxicol. 23, 1234–1244 (2010).
(59) S. Balbo, L. Meng, R.L. Bliss, J.A. Jensen, D.K. Hatsukami, and S.S. Hecht, Cancer Epidemiol. BiomarkersPrev. 21, 601–608 (2012).
(60) B.H. Yun, V. Sidorenko, T.A. Rosenquist, K.G. Dickman, A.P. Grollman, and R.J. Turesky, Toxicol. Res. 4, 763–776 (2015).
Jingshu Guo and Robert J. Turesky are with the Masonic Cancer Center and Department of Medicinal Chemistry at the University of Minnesota in Minneapolis, Minnesota. Peter W. Villalta is with the Masonic Cancer Center in Minneapolis, Minnesota. Direct correspondence to: rturesky@umn.edu

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.