Effect of Tissue Optical Properties on the Fluorescence of BODIPY Derivative as a Photosensitizer for Photodynamic Therapy
Photodynamic therapy is widely used as an established biomedical optical modality for the conservative treatment of tumors. This work investigates laser-induced fluorescence spectroscopy of the emerging photodynamic photosensitizer BODIPY-520 in turbid media. A 520 nm laser diode was used for exciting tissue-like optical phantoms. Effects of scattering and absorption of the turbid medium on the recorded fluoresced signal were studied. The results show that the BODIPY-520 photosensitizer has a prominent fluorescence peak at 600 nm, and that the optical properties of the surrounding turbid medium influence the spectral characteristics of fluorescence signals. The experimental work reveals that the characteristic fluorescence intensity peak of BODIPY-520 photosensitizer positioned at 600 nm is red-shifted to 675 nm in the presence of absorbing component, and with no red-shift in the presence of scattering components in the optical phantom under investigation. The effect of oxygen concentration on the detected fluorescence spectra is also quantified. The findings obtained from this study can be incorporated into an optimized protocol for BODIPY-520 based photodynamic therapy.
Photodynamic therapy (PDT) is an emerging treatment method for treating tumors especially malignant tumors (1). PDT, as a non-invasive therapeutic strategy, depends on toxic selective activity for the oncological cell (2). Basically, PDT involves three primary components: a photosensitizer, a light source, and oxygen. The photosensitizer is accumulated in diseased tissues. Then, after some time, the photosensitizer is activated with a light source of an appropriate wavelength in the presence of molecular oxygen present in tissues. This leads to two types of operations—type 1 and type 2. In both cases, reactive oxygen species (ROS) induce cell death by apoptosis or necrosis, thus making PDT interesting for the treatment of several diseases, and, more importantly, in a more conservative manner in comparison with other techniques that are commonly used in oncology (3). Over the last three decades, different generations of photosensitizers have been used for photodynamic therapy in an erroneous number of oncological applications. Regardless of the type (whether from the first, second, or third generation), a good photosensitizer is expected to show strong absorption in the visible range of wavelength, effective intersystem crossing (ISC), high singlet oxygen quantum yield, and tissue penetration (4). Despite the extensive research conducted to develop new and improved photosensitizers, with desirable properties, creating what can be considered the “best” photosensitizer remains a big challenge for the pharmaceutical industry. However, recent studies have focused mainly on chlorine, phthalocyanine, and difluoroboron dipyrromethene- (BODIPY-) derivatives, aiming to provide a better understanding of the factors affecting the efficacy of photosensitizer molecules (5).
Recently, the BODIPY derivative (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) as a photosensitizer has attracted the attention of many research studies (6,7). The main outstanding characteristic of BODIPY derivatives is their chemical versatility and excellent physical characteristics (8), including their exhibiting broadband spectral features in the ultraviolet-visible (UV-vis) region of the electromagnetic spectrum which even extend to the near-infrared (NIR). Moreover, BODIPY derivatives have been utilized in many applications in different fields mainly in dye laser technology, development of fluorescent probes and sensors, artificial antennae, photosensitizers in solar cells, and photosensitizers in photodynamic therapy (9). In a study by Killoran and coworkers in 2002, the possibility of using BODIPY derivatives as efficient photosensitizers for photodynamic therapy (PDT) was demonstrated (10,11).
As for oxygen, it is well known that normal human cells need just the right amount of oxygen to stay healthy. The relationship between cancer cells and oxygen is complicated. Nevertheless, the difference in oxygen levels between cancer cells and normal cells continues to motivate research. For example, some companies have tried to develop drugs that are activated in the hypoxic environment of tumors, but which would not be active in normal cells.
In addition, a growing number of optical techniques have been used to improve the performance of optical technologies used in medical therapy. For instance, fluorescence measurements can provide insightful information for biomedical optical applications, such as the detection of cancerous or dysplastic lesions, and, more importantly, photosensitizer dosimetry through photodynamic therapy (12,13).
The morphological features of the fluorescence spectrum contain valuable information on fluorophores in the measurement volume, including their concentration, and the local environment. The interrogation of fluorescent agents in different tissue types is one of the key applications of fluorescence spectroscopy, and has been widely incorporated in PDT research, as well as in clinical practice. Several prior studies (14) confirmed that spectral features of photosensitizers used in PDT can be varied during the therapy, and thus the collected fluorescence can be seen as a reliable marker for the efficiency of the photodynamic therapy.
However, biological tissue is a turbid medium featuring scattering and absorption events that affect the fluorescence measurements in biological tissue of interest (10), thus complicating the quantification of fluorescence due to the influence of tissue optical properties on the collected fluorescence spectrum (15). Different approaches have been taken to remove the influence of the tissue optical properties on the detected fluorescence. Most of these approaches are based on semi-empirical models that are dependent in turn on the measuring device and the measurement geometry (16). Single-fiber (17) and multi-diameter single-fiber (18) fluorescence spectroscopy systems have been investigated to correct the effect of absorption and scattering on the collected fluorescent signals.
There have been many studies on the use of BODIPY derivatives as photosensitizers in PDT. However, most of them focus on the structural modification of the BODIPY core that leads to changes in the photophysical properties of the photosensitizer (19). However, in this work, we have selected a derivative of the BODIPY family known as 2,8-diethyl-1,3,5,7-tetramethyl-9-phenylbipyrromethene difluoroborate, which we call the BODIPY-520. To the best of our knowledge, the effect of surrounding optical properties on the fluorescence emanating from the BODIPY-520 photosensitizers has not been investigated, hence the independent effect of optical scattering and absorption coefficients need to be independently quantified.
Therefore, the main objective of this work is to experimentally investigate the effect of scattering and absorption coefficients on the spectral characteristics of fluorescence spectroscopy inside tissue simulating optical phantoms containing BODIPY-520 as a potential photosensitizer for PDT.
Materials and Methods
Sample Preparation
All samples used in this work have a size of 3 mL. The concentration of the BODIPY-520 photosensitizer was set to 2000 µM in initial fluorescent measurements, and 50 µM in all samples otherwise. We used 2, 8-diethyl-1,3,5,7-tetramethyl-9-phenylbipyrromethene difluoroborate (BODIPY-520) as a photosensitizer (795526, Sigma-Aldrich Inc.) that was prepared as a powder in a solution of methanol and phosphate buffered saline (PBS), and stored in the dark at 4 °C.
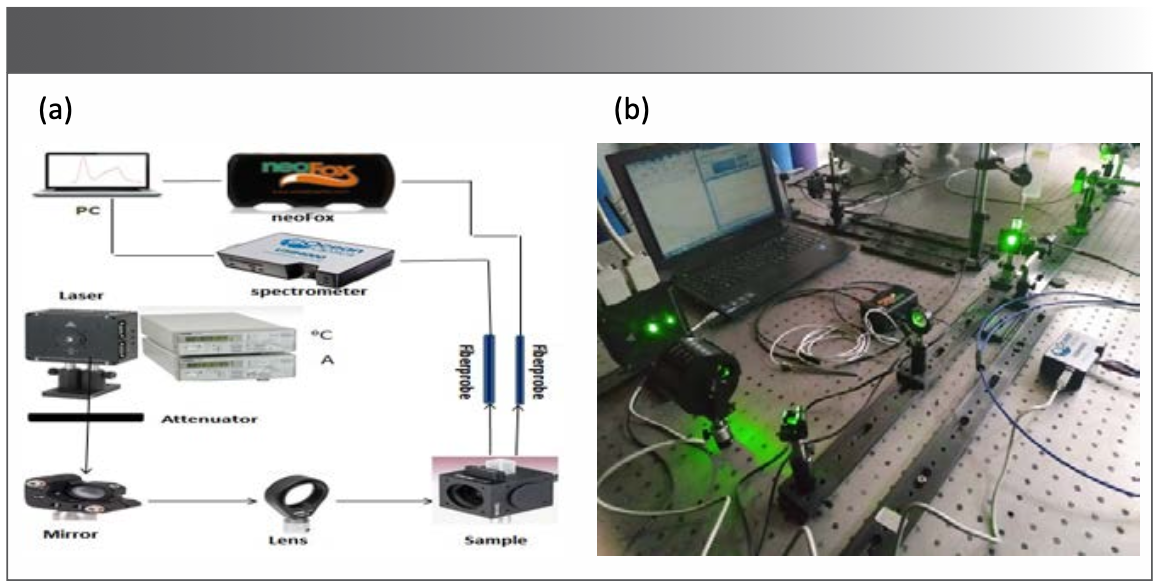
A schematic drawing representing the optical instrumentation for fluorescence measurements is depicted in equation 1. The light source used in this study was a semiconductor continuous laser with a wavelength of 520 nm (L520P50, Thorlabs Inc.) that was used as an excitation light source; the laser current and temperature were controlled with a temperature control unit (TED200C, Thorlabs Inc.) and a current control unit (LDC205C, Thorlabs Inc.).
Experimental Setup
The temperature and the current intensity were set at 40 oC and 150 mA, respectively. In addition, the power of the laser source can be adjusted up to 50 mW. A proper attenuator was used to set the power of the laser exciting the sample at 5 mW, followed by a proper mirror (PF05-03-M01, Thorlabs Inc.). For illuminating the entire sample, a lens was inserted to expand the excitation light over the entire target sample.
For collecting the emitted fluorescence signal, a collection fiber (Ocean Optics Inc.) with a diameter of 400 mm was used. The fiber is coupled to a miniature spectrometer (USB4000 FL, Ocean Optics Inc.). The spectrometer is connected via USB cable to a computer for acquiring fluorescence spectra. The recoding of data and signal processing of the collected spectra were controlled by Spectra suite software (Ocean Optics Inc.).
Oxygen measurements were also carried out in parallel with fluorescence measurements. To that end, a miniature oxygen meter (NeoFox, Ocean Optics, Inc.) was used to measure the oxygen concentration through a special collection fiber (Ocean Optics Inc.), as depicted in Figure 1; oxygen level was recorded as a percentage with a NeoFox Viewer Software (Ocean Optics, Inc.).
FIGURE 1: (a) Schematic diagram of the optical setup used for recording fluorescence spectra emanated from BODIPY-520 photosensitizer in tissue-like optical phantoms; (b) Overview of the optical setup.
The percentage of oxygen in the solution was increased or decreased by inserting bubbles of pure oxygen or nitrogen inside the air-saturated samples, respectively. Our oxygen sensor depends on the fluorescence of a chemical complex in a sol-gel substance located at the tip of optical fiber. When excited by a light-emitting diode (LED) at 475 nm, that light is delivered by optical fibers to the chemical complex at the tip of the probe. As a result, emitting fluorescence at 620 nm can be measured, and, through phase fluorometry (20), the phase shift between the excitation (LED) and emission (fluorescence) can be determined; consequently, the oxygen concentration can be estimated.
The sample was placed in a standard quartz cuvette (12.5 x 12.5 mm, with 10 mm light path) mounted on a cuvette holder (CVH100/M, Thorlabs Inc.), and closed tightly with rubber caps. The source–sample–detector geometry was maintained at the same position during measurements.
All experiments were conducted in a laboratory setting at room temperature and all values were presented as mean and standard deviation. The data were processed and graphed using Origin 8.0 software (Origin Lab Corporation) and Prism 8 (Graphpad Prism 8).
Optical Phantom
Optical phantoms were constructed in this work mainly to investigate the effect of scattering and absorption on the fluorescence of the BODIPY-520 photosensitizer. Optical phantoms were made of absorbing and scattering materials; that is, the optical phantom was made of Indian ink as an absorber (21), and intralipid as a scatterer (22). The optical properties of these materials were chosen so that they mimic the optical properties of biological tissues.
To quantify the effect of optical properties on the fluorescent intensity, three groups of optical phantoms were prepared. The first group contained six samples in which we varied the scattering coefficient into the following range of values (100, 80, 60, 40, 20, and 5)/mm, while the absorption coefficient was fixed at the value of 0.90/mm. The second group contained five samples in which the scattering coefficient was varied into the following range of values (60, 40, 20, 5, and 2)/mm, without adding absorbing materials. The third group contained five samples in which we varied the absorption coefficient into the following range of values (0.5, 0.8, 0.9, 5, and 10)/mm, whereas the scattering coefficient was fixed at the value of 5/mm.
The optical properties of the phantom were determined (both absorption and scattering) using the following equations 1 and 2.
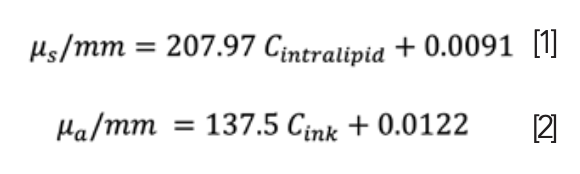
where the value of the absorbing or scattered laboratory has been compensated for; therefore, the concentration corresponding to the absorption or scattering coefficient was calculated, followed by a calculation of the ink or intralipid volume to be added when preparing the phantom (23). All previous samples were oxygenated at the following values: (60, 50, 40, 30, 20, 14, 10, 6, and 3) O2%. In addition, all measurements were performed in a dark laboratory environment at room temperature.
Results
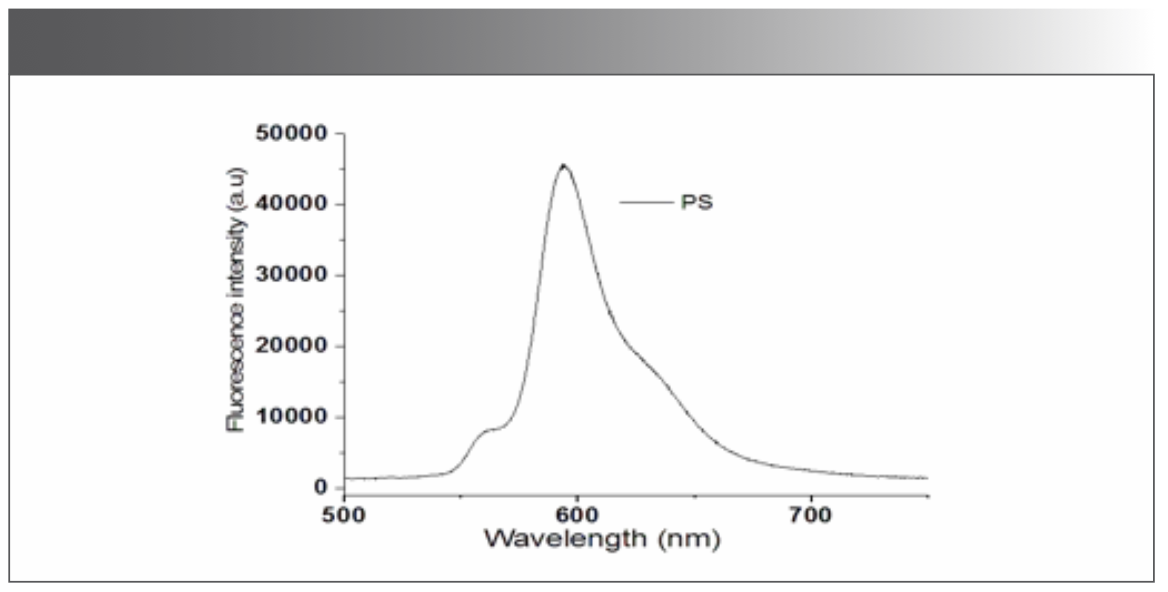
As an initial measurement, the fluorescence of the BODIPY-520 photosensitizer itself (diluted with methanol) was investigated. The excitation was performed with a 520 nm laser source. Figure 2 presents the fluorescence signal of the photosensitizer in the wavelength range from 500 to 800 nm. One can notice a prominent peak around 600 nm corresponding to the emission of the BODIPY-520 photosensitizer.
FIGURE 2: Recording of fluorescence spectra for BODIPY-520 photosensitizer.
Effect of Scattering on BODIPY-520 Fluorescence
To investigate the effect of scattering on the spectral features of the fluorescence spectra of the BODIPY photosensitizer, Intralipid 20% (I141, Sigma-Aldrich) was used as a scatterer, with varied concentrations as described above, corresponding to a set of scattering coefficients ranging from 5 to 100/mm.
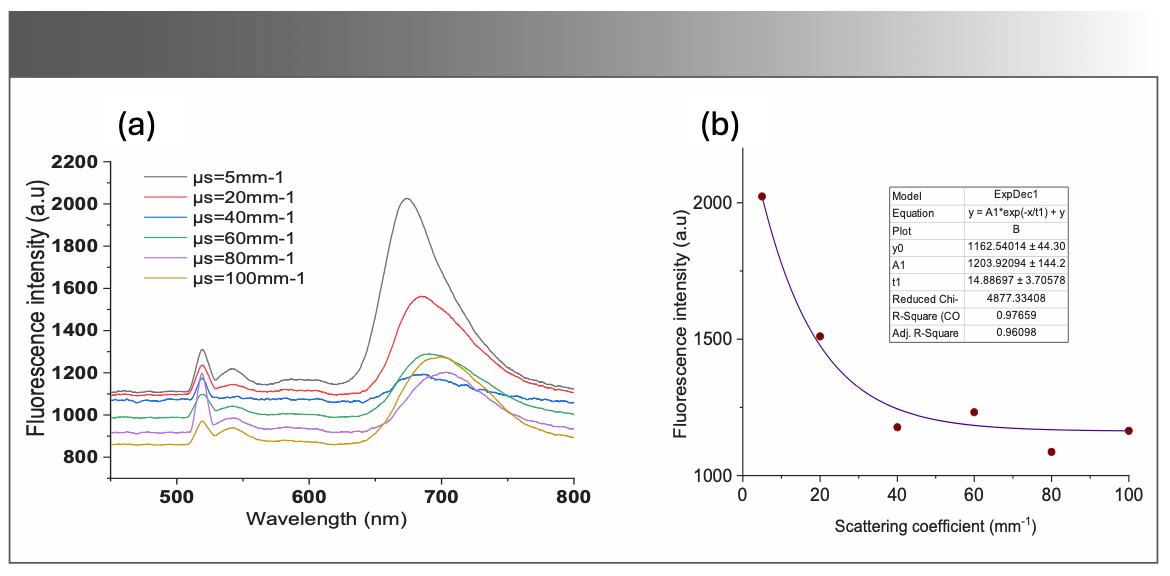
Figure 3a displays the measured fluorescence intensity of the BODIPY photosensitizer as a function of the scattering coefficient. The fluorescence intensity is inversely proportional to the scattering coefficient. One can also notice the spectral shift of the fluorescence peak to 675 nm. The fluorescence intensity of BODIPY photosensitizer at 675 nm was plotted as a function of the scattering coefficient values. This nonlinear trend in intensity can be fitted exponentially, as depicted in Figure 3b.
FIGURE 3: (a) Recordings of fluorescence spectra of the BODIPY-520 in the optical phantom for different scattering coefficients; (b) the fitted curve shows the exponential behavior of the fluorescence intensity (at 675 nm) as a function of the scattering coefficient.
Effect of Scattering on BODIPY-520 Fluorescence Without Absorbing Materials
To investigate the effect of scattering on the fluorescence spectra of the BODIPY photosensitizer without adding absorbing materials, four samples, optical phantoms, were prepared with four values of scattering coefficient in the range of 2 to 60/mm.
Figure 4a displays the measured fluorescence intensity of the BODIPY photosensitizer as a function of the scattering coefficient without absorbing agents. Apparently, the fluorescence intensity is inversely proportional to the scattering coefficient. One can also notice a prominent peak around 600 nm, as depicted in Figure 4a. This nonlinear trend in intensity can be fitted exponentially, as depicted in Figure 4b, and, therefore, there is no red shift due to introducing scattering materials into the BODIPY-520 optical phantom.
FIGURE 4: (a) Recordings of fluorescence spectra of the BODIPY-520 in the optical phantom without absorbing materials for different scattering coefficients; (b) Fluorescence intensity at 600 nm, the fitted curve shows the exponential behavior of the fluorescence intensity as a function of the scattering coefficient.
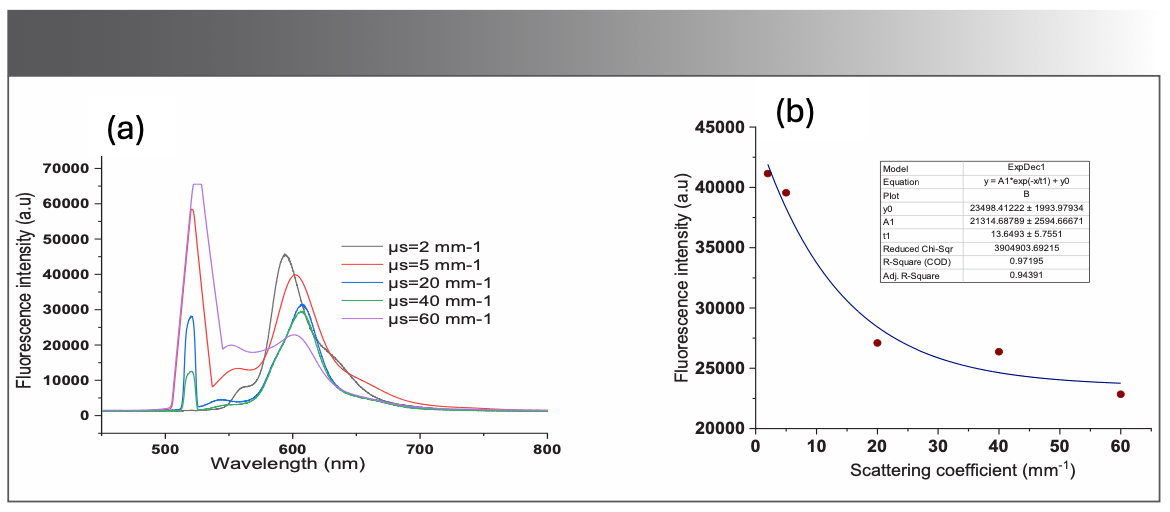
Effect of Absorption on BODIPY-520 Fluorescence
The impact of absorption on the fluorescence of the BODIPY photosensitizer was also quantified. Figure 5a shows the measured fluorescence spectra in the wavelength from 450 to 800 nm for different values of absorption coefficients. It is evident that the fluorescence intensity of the photosensitizer is inversely proportional to the absorption coefficient. Fluorescence intensity at 675 nm is plotted as a function of absorption coefficient, as displayed in Figure 5b.
FIGURE 5: (a) Recordings of fluorescence spectra of the BODIPY-520 in the optical phantom for different absorption coefficients; (b) Fluorescence intensity at 675 nm, the fitted curve shows the exponential behavior of the fluorescence intensity as a function of the absorption coefficients.
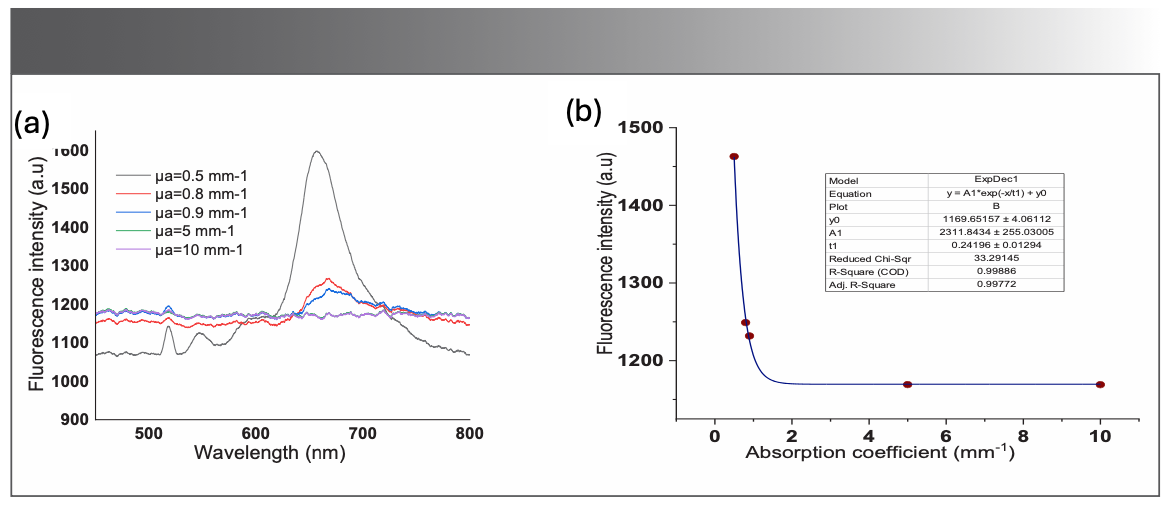
One can notice from Figure 5a that the fluorescence intensity declines rapidly for low values of absorption coefficient particularly for absorption coefficients lower than 1/mm. For values that are larger than 1/mm, the fluorescence intensity seems to be stable and constant. This can be explained by the rapid increase in the photobleaching in the sample, due to increased absorption.
Fluorescence and Oxygen Concentration
Figure 6a shows an example of the fluorescence spectra acquired for different levels of oxygenation. Clearly, there can be seen several emission peaks in the spectra; however, the most distinctive peak is around 675 nm, demonstrating the presence of another high emission peak of BODIPY-520 photosensitizer besides the emission peak around 530 nm. In addition, the emission peak around 675 nm features the spectral characteristics of BODIPY-520 photosensitizer in the near infrared region. It can also be observed from Figure 6a that a high level of oxygenation in the sample leads to a higher fluorescence emission peak around 675 nm.
FIGURE 6: (a) Recordings of fluorescence spectra of the BODIPY-520 in the optical phantom for different levels of oxygen concentration in a sample with μs = 5/mm; (b) Fluorescence intensity at 675 nm, the fitted curve shows the linear trend of the BODIPY-520 fluorescence intensity as a function of oxygen concentration.
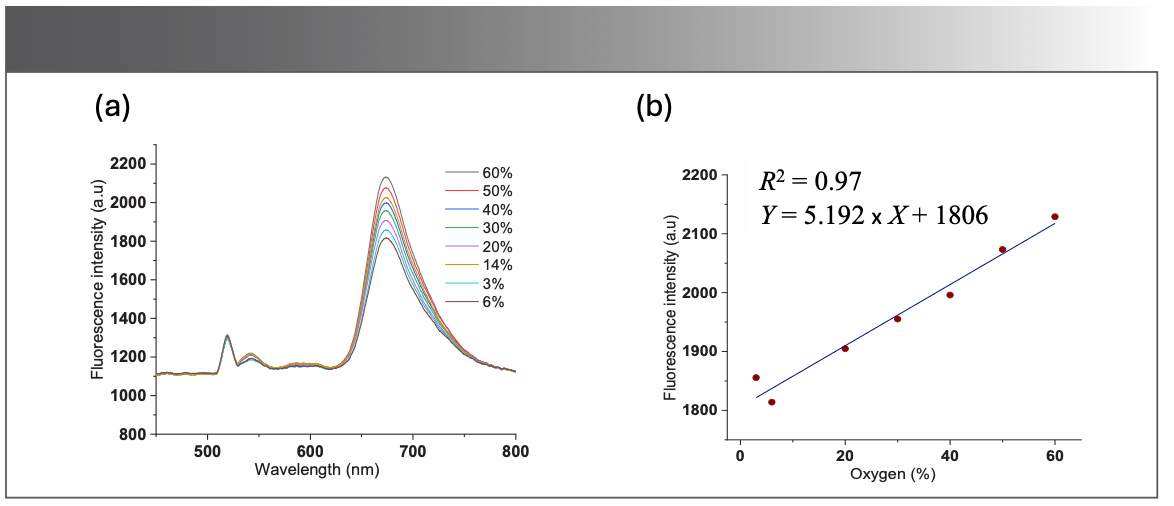
Fluorescence emission spectra were also measured for a sample with a scattering coefficient set at 5/mm, and for different values of oxygenation ranging from 6% to 60%. The emission peak around 675 nm was plotted against the corresponding oxygen concentration. One can see that the fluorescence intensity is directly proportional to oxygen concentration. This increase in intensity can be fitted linearly, as shown in Figure 6b.
Discussion
The results presented in this work showed the influence of surrounding tissue optical properties on the detected fluorescence of BODIPY-520 photosensitizer. The determination of the photosensitizer fluorescence is of great importance before, during, and after photodynamic therapy of tumorous tissue. Since photodynamic therapy highly depends on tissue oxygenation, changes in the oxygen saturation levels during PDT affect the optical properties of the tissue volume under investigation. Additionally, the blood volume fraction may also change and lead to variations in tissue absorption and scattering coefficients. Therefore, measuring the intrinsic fluorescence of the PDT photosensitizer can provide valuable feedback on the tissue optical properties.
Furthermore, the results of this study have shown that increased oxygen concentration leads to an increase in fluorescence intensity, however, the variation in fluorescence due to the presence of oxygen is relatively small in comparison with the change in fluorescence intensity due to variation in absorption and scattering coefficients. Since the goal of this work is focused on the effect of scattering and absorption coefficients on the BODIPY-520 photosensitizer fluorescence, the effect of oxygen concentration on the fluorescence intensity might be important when linked to the singlet oxygen measurements related to PDT (24).
Our findings regarding variations in spectral shape and intensity of the are in agreement with previous studies (25,26) that found a considerable influence of tissue optical properties on the fluorescence intensity and spectral shape of chlorine, a widely investigated photosensitizer. Another widely used photosensitizer in PDT is protoporphyrin IX (Ppix). Our results are also in agreement with a study by Anand and associates (27). Moreover, our results are consistent with another study (28) showing that the presence of absorbing and scattering materials leads to a shift in the fluorescence peak of the photosensitive despite the use of different solvents.
Another point to consider is the remarkable shift in fluorescence intensity peak by 75 nm towards higher wavelengths (that is, around 675 nm). This red shift is due to introducing aborting or scattering materials into the BODIPY-520 optical phantom. These observations in the spectral shift are in agreement with prior studies that investigated other photosensitizers used in PDT. Furthermore, van Leeuwen-van Zaane and associates (15) found significant differences in spectral shape over time between ce6 and Bremachlorin photosensitizers. They also observed a shift to the red in the wavelength position of the fluorescence intensity maximum for ce6 photosensitizer, whereas a blue shift was observed for Bremachlorin photosensitizers.
An important finding from this work is that, unlike previous studies, the variation in spectral features of BODIPY-520 photosensitizer fluorescence was investigated for a set of absorption and scattering values, and, therefore, a relationship between measured fluorescence can be determined as a function of tissue optical properties. Another significant observation for this study is that the impact of absorbing agents on the fluorescence of BODIPY-520 photosensitizer is more profound than the scattering agents, which could provide more information about the medium in terms of scattering and absorption from the fluorescence spectrum of BODIPY-520 photosensitizer.
One drawback of this study is the use of fluorescence spectroscopy without applying optical filtering of the fluorescence emission or excitation. For the purpose of this study, the detected fluorescence spectra were easily discernible; however, filtering could be required for in vivo applications where the fluorescence spectra might be oppressed by other fluorophores in the biological tissue of interest.
Despite this limitation, the presented experimental setup provides a capability to evaluate fluorescence spectra of BODIPY derivative for a wide range of optical properties that cover both intact and diseased tissue in clinical practice. However, future work may explore the difference between two separate optical phantoms with optical properties representing healthy and malignant tissue respectively.
Conclusion
This paper experimentally investigates the fluorescence spectroscopy of the BODIPY-520 photosensitizer. It also evaluated the effect of the optical properties of the medium on the fluorescence of the BODIPY-520 photosensitizer used in photodynamic therapy. The results demonstrated the presence of distinctive fluorescence emission at around 675 nm. This has an important implication for using BODIPY-520 photosensitizer as a potential fluorophore with emission spectrum in the near infrared spectrum.
The findings also revealed that both absorption coefficient and scattering have a profound impact on the intensity of fluorescence. This has potential applications in biomedical optical measurements related to the fluorescence measurements during photodynamic therapy of tumors—that is, rendering the monitoring of photosensitizer during photodynamic therapy more effective, and consequently improved photodynamic therapy overall. This study can be also extended to investigate other optical properties associated with specific biological tumorous tissue, and this, in turn, would further improve the accuracy of BODIPY-520 fluorescence-based biomedical optical diagnostics.
Funding
This work was funded by Damascus University.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Availability of Data and Material
Data are available from authors upon request.
Author Contribution Statement
All authors contributed equally to the paper.
References
(1) Agostinis, P.; Berg, K.; Cengel, A. K.; Foster, T. H.; Girotti, A. W.; Gollnick, S. O.; Hahn, S. M.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61 (4), 250–281. DOI: 10.3322/caac.20114
(2) Celli, J. P.; Spring, B. Q.; Rizvi, I.; Evans, C. L.; Samkoe, K. S.; Verma, S.; Pogue, B. W.; Hasan. T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110 (5), 2795–2838. DOI: 10.1021/cr900300p
(3) Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pract. 2015, 24, 14–28. DOI: 10.1159/000362416
(4) Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells. Chem. Soc. Rev. 2015, 44 (14), 4953–4972. DOI: 10.1039/c5cs00030k
(5) Zhang, J.; Jiang, C.; Longo, I. P. F.; Azevedo, R. B.; .Zhang, H.; Muehlmann, L. A. An Updated Overview on the Development of New Photosensitizers for Anticancer Photodynamic Therapy. Acta Pharm. Sin. B. 2018, 8(2), 137–146. DOI: 10.1016/j.apsb.2017.09.003
(6) Abrahamse, H.; Hamblin, M. R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473 (4), 347–364. DOI: 10.1042/BJ20150942
(7) Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet Photosensitizers: From Molecular Design to Applications. Chem. Soc. Rev. 2013, 42 (12), 5323–5351. DOI: 10.1039/c3cs35531d
(8) A. Kamkaew, S.H. Lim, H.B. Lee, L.V. Kiew, L.Y. Chung, and K. Burgess. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42 (1), 77–88. DOI: 10.1039/c2cs35216h
(9) Staudinger, C.; Breininger, J.; Klimant, I.; Borisov, S. M. Near-Infrared Fluorescent Aza-BODIPY Dyes for Sensing and Imaging of pH from the Neutral to Highly Alkaline Range. Analyst 2019, 144 (7), 2393–2402. DOI: 10.1039/c9an00118b
(10) Killoran, J.; Allen, L.; Gallagher, J. F.; Gallagher, W. M.; Donal, F. O. Synthesis of BF2 Chelates of Tetraarylazadipyrromethenes and Evidence for their Photodynamic Therapeutic Behaviour. Chem. Commun. 2002, 17, 1862–1863. DOI: 10.1039/B204317C
(11) Gorman, A.; Killoran, J.; O’Shea, C.; Kenna, T.; Gallagher, W. M.; O’Shea, D. F. In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy. J. Am. Chem. Soc. 2004, 126 (34), 10619–10631. DOI: 10.1021/ja047649e
(12) Marcu, L. Fluorescence Lifetime Techniques in Medical Applications. Ann. Biomed. Eng. 2012, 40 (2), 304–331. DOI: 10.1007/s10439-011-0495-y
(13) Diamond, K. R.; Patterson, M. S.; Farrell, T. Quantification of Fluorophore Concentration in Tissue-Simulating Media by Fluorescence Measurements with a Single Optical Fiber. J. Appl. Opt. 2003, 42 (13), 2436–2442. DOI: 10.1364/ao.42.002436
(14) Koning, S. G.; .Baltussen, J. M.; Karakullukcu, M. B.; Dashtbozorg, B.; Smit, L. A.; Dirven, R.; Hendriks, B. H. W.; Sterenborg, H. J. C. M.; and Ruers, T. J. M. Toward Complete Oral Cavity Cancer Resection Using a Handheld Diffuse Reflectance Spectroscopy Probe. J. Biomed. Opt. 2018, 23 (12), 121611. DOI: 10.1117/1.jbo.23.12.121611
(15) van Leeuwen-van Zaane, F.; Gamm, U. A.; van Driel, P. B.; Snoeks, T. J.; Brujin, H. S.; Sterenborg, H. J.; Robinson, D. J. Intrinsic Photosensitizer Fluorescence Measured Using Multi-diameter Single-Fiber Spectroscopy in vivo. J. Biomed. Opt. 2014, 19 (1), 015010. DOI: 10.1117/1.jbo.19.1.015010
(16) Stepp, H.; Beck, T.; Beyer, W.; Pfaller, C.; Schuppler, M.; Sroka, R.; Baumgartner, R. Measurement of Fluorophore Concentration in Turbid Media by a Single Optical Fiber. Med. Laser Appl. 2007, 22 (1), 23–34. DOI: 10.1016/j.mla.2006.08.005
(17) Amelink, A.; Kruijt, B.; Robinson, D. J.; Sterenborg, H. J. Quantitative Fluorescence Spectroscopy in Turbid Media Using Fluorescence Differential Path Length Spectroscopy. J. Biomed. Opt. 2008, 13 (5), 054051. DOI: 10.1117/1.2992132
(18) Gamm, U. A.; Kanick, S. C.; Sterenborg, H. J. C.; Robinson, D. J.; Amelink, A. Measurement of Tissue Scattering Properties Using Multi-diameter Single Fiber Reflectance Spectroscopy: in silico Sensitivity Analysis. Biomed. Opt. Express 2011, 2 (11), 3150–3166. DOI: 10.1364/boe.2.003150
(19) Awuah, S. G.; You, Y. Boron Dipyrromethene (BODIPY)-Based Photosensitizers for Photodynamic Therapy. RSC Advances 2012, 2 (30), 11169–11183. DOI: 10.1039/c2ra21404k
(20) Boehme, S.; Duenges, B.; Klein, K. U.; Hartwich, V.; Mayr, B.; Consiglio, J.; Baumgardner, J. E.; Markstaller, K.; Basciani, R.; Vogt, A. Multi Frequency Phase Fluorimetry (MFPF) for Oxygen Partial Pressure Measurement: Ex vivo Validation by Polarographic Clark-Type Electrode. PLoS One. 2013, 8 (4), e60591. DOI: 10.1371/journal.pone.0060591
(21) Di Ninni, P.; Martelli, F.; Zaccanti, G. The Use of India Ink in Tissue-Simulating Phantoms. Opt. Express 2010, 18 (26), 26854–26865. DOI: 10.1364/oe.18.026854
(22) Aernouts, B.; Van Beers, R.; Watté, R.; Huybrechts, T.; Lammertyn, J.; Saeys, W. Visible and Near-Infrared Bulk Optical Properties of Raw Milk. J. Dairy Sci. 2015, 98 (10), 6727–6738. DOI: 10.3168/jds.2015-9630
(23) Shahin, A.; Bachir, W. Broadband Spectroscopy for Characterization of Tissue-Like Phantom Optical Properties. Polish J. Med. Phys. Eng. 2017, 23 (4), 121. DOI: 10.1515/pjmpe-2017-0020
(24) Chen, L.; Lin, L.; Li, Y.; Lin, H.; Qiu, Z.; Gu, Y.; Li, B. Effect of Oxygen Concentration on Singlet Oxygen Luminescence Detection. J. Lumin. 2014, 152, 98–102. DOI: 10.1016/j.jlumin.2013.10.034
(25) Kanick, S. C.; Robinson, D. J.; Sterenborg, H. J. C. M.; Amelink, A. Extraction of Intrinsic Fluorescence from Single Fiber Fluorescence Measurements on a Turbid Medium Opt. Lett. 2012, 37 (5), 948–950. DOI: 10.1364/ol.37.000948
(26) Kanick, S. C.; Robinson, D. J.; Sterenborg, H. J. C. M.; Amelink, A. Semi-Empirical Model of the Effect of Scattering on Single Fiber Fluorescence Intensity Measured on a Turbid Medium. Biomed. Opt. Express 2012, 3 (1), 137–152. DOI: 10.1364/boe.3.000137
(27) Suresh Anand, B. S.; Sujatha, N. Effects of Intralipid-10% in Fluorescence Distortion Studies on Liquid-Tissue Phantoms in UV Range. J. Biophotonics 2011, 4, 92–97. DOI: 10.1002/jbio.200900096
(28) Kamel, B.; Sayem El-Daher, M.; Bachir, W.; Ibrahim, A.; Aljalali, S. Effect of Solvents on the Fluorescent Spectroscopy of BODIPY-520 Derivative. J. Spectrosc. (London, U. K.) 2022, 6, 1172183. DOI: 10.1155/2022/1172183
Buthaina Kamel and Wesam Bachir are with the Biomedical Photonics Laboratory, Higher Institute for Laser Research and Applications at Damascus University, in Damascus, Syria. Moustafa Sayem El-Daher, Sinan Aljalali, and Kamel are with the Laser Spectroscopy Laboratory, Higher Institute for Laser Research and Applications at Damascus University, in Damascus, Syria. El-Daher is also part of the Faculty of Civil Engineering at Arab International University, in Damascus, Syria. Bachir is also part of the Institute of Metrology and Biomedical Engineering, Faculty of Mechatronics at the Warsaw University of Technology, in Warsaw, Poland. Direct correspondence to wesam.bachir@pw.edu.pl
Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.