Efficient Removal of Polyatomic Spectral Interferences for the Multielement Analysis of Complex Human Biological Samples by ICP-MS
How to reduce plasma-, matrix-, and solvent-induced polyatomic interference by bleeding a collision–reaction gas into the tip of the interface skimmer cone

This column installment presents a novel approach to reduce plasma-, matrix-, and solvent-induced polyatomic interferences by bleeding a collision–reaction gas into the tip of the interface skimmer cone. This approach improves the detection capability of notoriously difficult elements such as Cr, V, and As in human blood samples. In addition, data will be presented for the optimization of operating parameters for the determination of ultratrace levels of beryllium in human urine.
Inductively coupled plasma–mass spectrometry (ICP-MS) using traditional quadrupole mass analyzers has been extensively used over the past two decades for the determination of trace and ultratrace elements in human body fluids (1). This technique offers multielemental capabilities, ease of use, simplified sample preparation, and improved detection limits compared to traditional atomic spectroscopy techniques like graphite furnace atomic absorption (GFAA) and inductively coupled plasma–optical emission spectroscopy (ICP-OES). However, this approach is prone to several interferences that, if not handled correctly, can impact the detection capability for many of the critical elements.
For example, high levels of urea, protein, fat, sodium, and chloride ions in urine can cause suppression or enhancements of analytes being determined by ICP-MS. In addition, with blood and urine samples, there is the potential for signal drift caused by these biological, carbonaceous components being deposited on the interface cones and ion lens system, which often necessitates large dilutions. But probably the most severe instrumental problem is that major and trace elements in the sample can combine with argon-, solvent-, and acid-based species to produce quite severe polyatomic-, isobaric-, and oxide-based spectral interferences (2).
This study evaluates the capability of a novel approach to reduce plasma-, matrix-, and solvent-induced polyatomic interferences by bleeding a collision–reaction gas into the tip of the interface skimmer cone to improve the detection limits of the notoriously difficult ICP-MS elements such as Cr, V, As, and Se in whole blood samples. In addition, it will present the benefits of high sensitivity with low background in generating very low method detection limits for beryllium in diluted human urine samples.
Spectral Interferences Associated with Biological Samples
Table I represents typical polyatomic spectral interferences associated with analyzing body fluids by ICP-MS, together with the analyte masses they impact.
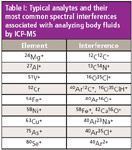
Table I: Typical analytes and their most common spectral interferences associated with analyzing body fluids by ICP-MS
Several methods have been used to reduce these spectral interferences, including mathematical correction equations, matrix elimination techniques, cool plasma conditions, high resolution spectrometers (3), and more recently, the use of collision–reaction cell technology. With this latter approach, ions enter the interface in the normal manner, and are then directed into a pressurized collision–reaction cell positioned prior to the analyzer quadrupole. A collision–reaction gas (for example, He, H2, NH3, CH4, or O2depending on the design) is then bled into the cell containing a multipole (a quadrupole, hexapole, or octapole), which has the effect of initiating collisions and reactions with the interfering species. By a number of different ion–molecule interaction mechanisms, polyatomic interfering ions will either be converted to harmless noninterfering species, or the analyte will be converted to another ion that is not interfered with (4).
Collision–Reaction Interface
An alternative approach to using a pressurized cell is to inject a collision–reaction gas (typically He or H2) at relatively high flow rates (100–150 mL/min) into the plasma through the apertures of the interface skimmer cone, where the plasma density is high (5). This approach increases the rate of interactions between the introduced gas and interfering ions and improves the attenuation of interfering ions. In addition, the collision–reaction gas is supplied directly to the plasma, which means that the plasma electrons are still available to assist in attenuating the interfering ions through electron–ion recombination. The presence of plasma electrons also significantly reduces the generation of secondary by-product ions produced from the interference attenuation process. The overall result is that most argon-based polyatomic interferences are destroyed or removed before they are extracted into the ion optics.
The instrument used in this study was an M90 ICP-MS system (Bruker Daltonics Inc.) fitted with a collision–reaction interface (CRI) to minimize many of the common spectral interferences encountered in human blood and urine samples. The instrument has been described in the literature, but is basically a high-sensitivity instrument that utilizes a 90° ion mirror and off-axis quadrupole to generate analyte signals in excess of 1 billion cps/ppm with very low background noise (6,7). The initial study focuses on finding the best collision–reaction gas to use, along with optimization of the gas flows to produce the highest analyte sensitivity with the lowest background contribution from the inteferent. These optimized conditions will then be used to determine background equivalent concentration (BEC) values for a suite of elements that include a combination of relatively straightforward analytes with no spectral background and a group of analytes that are notoriously difficult to determine in human body fluids. Finally, these optimized parameters are used to analyze two freeze-dried blood certified reference materials (8).
Optimization of Operating Conditions
Operating conditions were optimized before sample analysis using a 100 µg/L solution of the multielement standard made up in a blank–diluent solution of 1% isopropanol, 0.01% ammonium salt of EDTA, 0.01% Triton-X 100 in 18-MΩ deionized water to simulate the matrix content of the samples. Plasma gas flows, sample introduction components, and ion optics parameters were tuned so that the analyte sensitivities were maximized while maintaining the background level signals close to zero. The optimized instrumental conditions used for this evaluation are given in Table II.

Table II: The instrumental operating conditions used for this evaluation
After the instrument was tuned for maximum sensitivity, the collision–reaction gas flows of both hydrogen and helium gases were optimized separately to get a better understanding of the capabilities of both gases to reduce the major interfering ions. The collision–reaction gas was introduced through the aperture of the skimmer cone and the sensitivities were monitored for the internal standard elements 45Sc, 89Y, and 115In, while monitoring two of the major interferences — 40Ar40Ar (on 80Se) and 40Ar12C (on 52Cr). Figure 1 demonstrates the optimization of hydrogen gas, showing signals as a function of hydrogen flow rate. It should be noted that there is no Se or Cr in this solution, so the signals at mass 80 and 52 amu are contributions from the 40Ar40Ar and 40Ar12C polyatomic ions, respectively. A sharper decrease of the interferent signals can be seen compared to those of the internal standards, showing evidence of the removal of the ArAr+ and ArC+ polyatomic interferences with increasing hydrogen flow rate. The optimization plot shows that at a flow rate of 140 mL/min, interferent signals decrease by six orders of magnitude but Sc, Y, and In are only reduced by two orders of magnitude.

Figure 1: Optimization of hydrogen gas flow rate, showing IS (Sc, In, and Y) signals while monitoring the interferences 40Ar40Ar (80Se) and 40Ar12C (52Cr).
The same tests where conducted using helium gas at the aperture of the skimmer cone. The effect of helium flow rate on the signal intensities is illustrated in Figure 2. It can be seen that even though there is a sharper decrease in the intensities of the interfering ions as the helium flow rate is increased, a signal reduction of only two orders of magnitude is observed at mass 80 and 52 amu at 140 mL/min — significantly less than what was observed when using hydrogen gas. This leads to the conclusion that hydrogen gas is more efficient than helium at removing these two argon-based interferences.
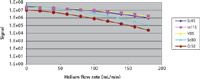
Figure 2: Optimization of helium gas flow rate, showing IS (Sc, In, and Y) signals while monitoring the interferences 40Ar40Ar (80Se) and 40Ar12C (52Cr).
To evaluate the impact of the gases on reducing the two major chloride-based interferences encountered in ICP-MS determinations of biological samples — 35Cl16O (on 51V) and 40Ar35Cl (on 75As) — the solutions from the previous test were spiked with 1% HCl, 100 mg/L of Ca, 200 mg/L of Na, K, and S, and 50 mg/L of Fe and Mg to simulate 1:10 diluted blood samples. In this case, the ratio of the interfering ion to the yttrium IS signal was monitored and the hydrogen and helium gas flows were increased. The optimization plots for the reduction of the 35Cl16O, and 40Ar35Cl interfering ions are shown in Figures 3 and 4, respectively. A decrease in the ratios is observed in both cases when hydrogen is used as a collision–reaction gas at the aperture of the skimmer cone. When helium is introduced, the ratio remains constant or even increases in the case of 35Cl16O, indicating that the slope of the interfering ion signal is similar or even less than that of the analyte signal. Again confirming that hydrogen is the better option for minimizing chloride based polyatomic interferences in this type of clinical matrix. However, it should be emphasized that when the detection limit requirements are not as demanding or when the interferences change based on a different matrix, helium has also shown itself to be a very efficient collision–reaction gas to use (9).
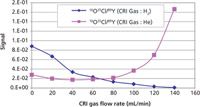
Figure 3: Hydrogen and helium gas optimization plots for the reduction of the 35Cl16O (51V) interfering ions in a simulated blood matrix.
Background Equivalent Concentrations
With this information, BEC values were determined on a multielement suite of analytes, consisting of a combination of relatively straightforward analytes (Mn, Co, Ni, Cd, Tl, Pb, and U) and a group of analytes that are notoriously difficult to determine by ICP-MS (V, Cr, Cu, As, and Se). The BECs were measured in the isopropanol–diluent solution using both standard (no gas) mode and CRI mode using hydrogen as the collision–reaction gas. The values, which are reported in Table III, show a significant improvement in background equivalent concentration for 51V, 52Cr, 75As, and 80Se when hydrogen is used at a flow rate of 140 mL/min, without compromising the BEC values for the other elements. This confirms that a single set of instrumental and CRI conditions can be used for the multielemental analysis of human blood samples. It's also worth noting that selenium was reported at three different isotopes, because very often the major isotope of 80 amu cannot be used because of the extremely large contribution from the argon dimer.
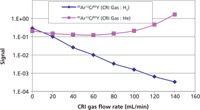
Figure 4: Hydrogen and helium gas optimization plots for the reduction of the 40Ar35Cl (75As) interfering ion in a simulated blood matrix.
Analysis of Whole Blood Reference Standards
To confirm these data in real samples, two freeze-dried seronorm whole blood (WB) certified reference materials (SERO AS) were reconstituted as recommended by the manufacturer and diluted 1:10 before analysis with 1% isopropanol, 0.01% ammonium salt of EDTA, and 0.01% Triton-X 100 in 18 MΩ deionized water. A four-point multielement calibration was carried out at trace, low, medium, and high analyte concentrations (depending on the level in the reference material) using a 10 µg/mL stock solution. Multielement analysis was performed on a low level (L1) and a high analyte level (L3) of the whole blood, to reflect the severity of the polyatomic spectral interferences on the difficult elements, especially V, Cr, As, and Se. The results are reported in Table IV. Upon further examination, it can be seen that the vast majority of the analyte measurements were well within the certified ranges, confirming that one set of conditions can be used for the full suite of analytes.

Table III: BEC values for standard elements compared to those for elements with a high spectral background, showing a significant improvement for 51V, 52Cr, 75As, and 80Se when H2 is used as the CRI gas, without compromising the BEC values for the other elements
Determination of Beryllium in Human Urine
Beryllium is widely used in the manufacture of ceramics, mirrors, automotive alloys, computer circuit boards, and various electrical components. It also occurs naturally in rocks, coal, soil, volcanic dust, and potable water systems. However, it is extremely toxic when absorbed through the lungs or the digestive system. For that reason, it has been classified as a human carcinogen by the World Heath Organization, based on excess lung and central nervous system cancers in studies of exposed workers. Long-term exposure to the metal can cause serious health problems in the form of chronic beryllium disease (CBD), which shows itself as fatigue, difficulty in breathing, persistent coughing, and severe weight loss. It can be measured in the bloodstream, but to better understand its toxicity effects on the human body a faster and more sensitive method is achieved by measuring beryllium directly in urine samples by ICP-MS (10).
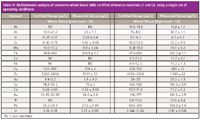
Table IV: Multielement analysis of seronorm whole blood (WB) certified reference materials L1 and L3, using a single set of operating conditions
Optimizing Beryllium Signal
However, one of the disadvantages of determining beryllium by ICP-MS is that it has a first ionization potential of approximately 9 eV, which means that only about 70% of beryllium is ionized in the plasma. And because it is monoisotopic, no other mass can be used for quantitation. Additionally, the determination of beryllium is further complicated by space charge effects, which means it can easily get "knocked out" of the ion beam by higher mass elements because it is a very light element (9 amu). However, these shortcomings can be overcome by optimizing instrument operating conditions to maximize the beryllium signal. It does not suffer from any major oxide interferences, so method optimization can be focused more around plasma power, nebulizer or sheath gas flow rates, and ion optics to generate the highest sensitivity for the lighter elements.
To achieve this, a 10-fold dilution of human urine was spiked to a 5-µg/L Be concentration. This dilution was then used to optimize the instrument to obtain the best signal for the light elements, keeping in mind that oxides and doubly charged ratios of greater than 3% were acceptable. By optimizing in this way, a signal of approximately 500,000 counts per second (cps) for the 5 µg/L Be (or 100 million cps/ppm) was achieved. This translated into a final method detection limit (MDL) of 0.3 ng/L in the 10-fold diluted urine samples. The optimized instrumental parameters for the determination of beryllium are shown in Table V.
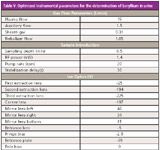
Table V: Optimized instrumental parameters for the determination of beryllium in urine
Preparation of Samples and Standards
Two ClinChek lyophilized (Ref 8849, Lot 923) urine control reference materials, Level I and Level II (Recipe, Chemicals and Instruments), were prepared as samples to confirm that this is achievable in real urine samples. As the samples were not acid digested, the method of standard additions was chosen to minimize any matrix effects. From the stock 100-µg/mL Be solution, an intermediate solution of 0.5 µg/L Be in 1% nitric acid was made from which a "standard additions" calibration curve of 0, 5, 10, 20, 50, and 100 ng/L was generated using the human urine sample diluted 10-fold to reduce effects from total dissolved solids. Additionally, 100 µg/L of 6Li internal standard was added automatically on-line to compensate for any sample transport effects. The standard additions calibration curve is shown in Figure 5. The correlation coefficient of 0.99975 indicates excellent linearity over the calibration range of 0–100 ng/L Be. The concentration of the Level I and II calculated from the standard additions plot is shown in Table VI, confirming very good agreement with the certified ranges for beryllium.

Figure 5: The standard additions calibration curve of 5, 10, 20, 50, and 100 ng/L Be spiked into a human urine sample.
In Summary
This work demonstrates the capability of ICP-MS with a collision–reaction interface for multielement analysis in complex matrices such as whole blood samples using a single set of instrument conditions. The traditionally problematic elements were measured with good accuracy and precision without compromising the performance of the other elements not suffering from interferences by using hydrogen as the collision–reaction gas through the aperture of the skimmer cone, which effectively removed the matrix and plasma-based interfering ions. Using one set of conditions minimizes the analysis time and also provides the advantage of low sample uptake for biological applications, where sample size is often a limitation. It should be emphasized that although hydrogen gas was found to be the optimum gas for the analyte levels in this matrix, in many other biological matrices, helium gas can also be an effective way of reducing the polyatomic interfering ions, particularly if the elemental detection limit requirements are less demanding.

Table VI: Beryllium concentrations (ng/L) in Level I and II ClinChek lyophilized urine control reference materials
In addition, the study has successfully demonstrated a very simple, fast, and effective way of carrying out the direct determination of beryllium in human urine samples, avoiding the traditional and time-consuming acid digestion sample preparation step. By optimizing instrumental parameters for maximum sensitivity for the lower mass elements and not being concerned about oxide or doubly charged species, a method detection limit of 0.3 ng/L Be was achieved in a 10-fold dilution of human urine, using the standard additions method.
Meike Hamester is the European director for ICP-MS for Bruker Daltonics at the Bruker ICP-MS Applications Laboratory in Berlin, Germany.

Meike Hamester
René Chemnitzer is an Application Scientist for ICP-MS for Bruker Daltonics at the Bruker ICP-MS Applications Laboratory in Berlin, Germany.

René Chemnitzer
Peio Riss is a European Application Specialist for Bruker Daltonics in Paris, France.

Peio Riss
Andrew Gaal is an ICP-MS Product Specialist for Bruker Biosciences in Melbourne, Australia.

Andrew Gaal
XueDong Wang is an ICP-MS Product Specialist for Bruker Biosciences in Melbourne, Australia.

XueDong Wang
Robert Thomas is a consultant and science writer specializing in trace element analysis.

Robert Thomas
Please direct correspondence to: Meike.Hamester@bdal.de.
References
(1) T.D.B. Lyon, G.S. Fell, R.C. Hutton, and A.N. Eaton, J. Anal. At. Spectrom. 3, 601 (1988).
(2) E. Pruszkowski, K. Neubauer, and R. Thomas, At. Spectrosc. 19(4), 111–115 (1998).
(3) N.M Reed, R.O. Cairns, R.C. Hutton, and Y. Takaku, J. Anal. At. Spectrom. 9, 881 (1994).
(4) P. Turner, T. Merren, J. Speakman, and C. Haines, Plasma Source Mass Spectrometry: Developments and Applications (The Royal Society of Chemistry, 1996), pp. 28–34.
(5) Bruker Daltonics Application Note CA-270111, "Principles and Performance of the Collision Reaction Interface for the aurora M90," http://www.bdal.com/uploads/media/CA-270111_Principles_and_performance_of_the_Collision_Reaction_Interface_for_the_aurora_M90.pdf.
(6) Bruker Daltonics CA-Application Note 270102, "A New Era in ICP-MS: Aurora M90," http://www.bdal.com/library/literature-room/detail-view/article/aurora-m90-a-new-era-in-icp-ms-3267/108.html.
(7) Bruker Daltonics Application Note CA-270112, "Innovative High Sensitivity 90-degree Reflecting ICP-MS Ion Optics for Routine Sample Analysis," http://www.bdal.com/uploads/media/CA-270112_Innovative_High_Sensitivity_90-degree_Reflecting_ICP-MS.pdf.
(8) Y. Abdelnour and J. Murphy, Varian 820-MS Application Note #28: "The Analysis of Whole Blood Samples by Collision Reaction Interface Inductively Coupled Plasma Mass Spectrometry."
(9) X.D. Wang, Application Note #CA-270105, "Direct Determination of Trace Elements in Urine Using the aurora M90 ICP-MS" http://www.bdal.com/uploads/media/CA-270105_Direct_Determination_of_Trace_Elements_in_Urine_using_the_aurora_M90.pdf.
(10) A. Gaal and P.-E. Riss, Application Note CA285182, "Analysis of Low Level Beryllium in Urine Using the aurora M90 ICP-MS," http://www.bdal.com/library/literature-room/detail-view/article/analysis-of-low-level-beryllium-in-urine-using-the-aurora-m90-icp-ms-4905/108.html.

Applications of Micro X-Ray Fluorescence Spectroscopy in Food and Agricultural Products
January 25th 2025In recent years, advances in X-ray optics and detectors have enabled the commercialization of laboratory μXRF spectrometers with spot sizes of ~3 to 30 μm that are suitable for routine imaging of element localization, which was previously only available with scanning electron microscopy (SEM-EDS). This new technique opens a variety of new μXRF applications in the food and agricultural sciences, which have the potential to provide researchers with valuable data that can enhance food safety, improve product consistency, and refine our understanding of the mechanisms of elemental uptake and homeostasis in agricultural crops. This month’s column takes a more detailed look at some of those application areas.