Emerging Trends and Opportunities in Discrete-Frequency Infrared and Raman Spectroscopic Imaging
Application Notebook
Recent advances in instrumentation have enabled new forms of vibrational chemical imaging, including discrete-frequency infrared (DFIR) microscopy and stimulated Raman scattering (SRS) microscopy. These technologies may represent a fundamental shift in how we approach spectroscopic imaging: Rather than collecting full spectra that contain redundant information, measuring a few important spectral frequencies may enable significant gains in speed, throughput, signal-to-noise ratio, and image quality. For IR microscopy, these advantages may be compounded by high-definition IR microscopy. Here we discuss recent advances in IR and nonlinear Raman imaging through the lens of "discrete-frequency" approaches and include several examples of applications and critical issues in instrumentation that are likely to be dominant research themes in the near future.
The importance of infrared (IR) and Raman spectroscopic imaging is strongly emerging in many disciplines in which knowledge of the spatial distribution of chemical constituents is of importance, including biomedical, polymers, forensics, and agricultural analyses (1–6). The traditional approach to spectroscopic analysis has been to record the entire spectrum at regular spatial intervals (7,8). In the simplest case, a spectrum is acquired at an illuminated point that is subsequently scanned over the entire area to be analyzed. The time required for data acquisition in this case increases at least linearly with the number of pixels. The use of multielement detectors, especially focal plane arrays (9,10) for IR imaging and charge-coupled devices for Raman imaging (11), provides a multichannel advantage to speed up data acquisition. Spreading light over large areas, the slow readout and poor electronics characteristic of thousands of detector elements often limit the signal-to-noise ratio (S/N) attained per pixel for an array detector in a specified time. The conduct of traditional spectroscopic measurements in an imaging format, hence, usually involves a gain in spatial specificity at the cost of a reduction in analytical performance in terms of S/N and acquisition time of a given sample volume. To combat the low S/N problem, nonlinear Raman spectroscopic methods were developed that provided an exceptional increase in signal. This increase arises from a pump-probe scheme in which a single or a few vibrational modes are interrogated at a time (6,12). Often, the desired information in a spatially heterogeneous sample can be obtained using just a few frequencies and the other parts of the spectrum are not needed. For example, two vibrational modes are sufficient to visualize the structure in a two-phase polymer blend (13) and tens of frequencies may be needed to extract histopathologic information from tissue samples. New technological advances along with the realization to only record specific frequencies that may be needed (14) has led to the emergence of the discrete-frequency infrared (DFIR) imaging (15) approach. Here, we review current technology with a special emphasis on recent developments in discrete-frequency imaging in both IR and Raman. We include several examples of applications and identify critical issues in instrumentation that are likely to be dominating research themes in the near future.
Infrared Spectroscopic Imaging
Overview of Recent Advances in Infrared Imaging
Recent advances in IR microscopic imaging have been in two broad areas: first, a better understanding of the relationship between image formation optics, which includes the recognition of optimal sampling rates leading to diffraction-limited measurements across the entire vibrational spectrum (high definition [HD]). Second is the advent and use of new light sources-in particular, narrow-bandwidth but widely tunable quantum cascade lasers (QCL). These advances, both individually and together, hold great promise for impact on the development of instrumentation in terms of imaging quality, acquisition speed, equipment price, and the potential for transition into a clinical environment. Figure 1 shows an example of the gain in image quality arising from the use of HD optics. The effective pixel size in this case is ~30-fold smaller than that in typical conventional optics. Without any additional modifications in hardware, the HD approach is limited by time constraints. To cover the same area, at least 30-fold higher time is required. Furthermore, a reduction in light intensity per pixel necessitates an additional increase in acquisition time to maintain S/N of data. Although the first attempts at HD imaging used a synchrotron source to decrease acquisition time by increasing light throughput (16), the emergence of broadly tunable QCLs offers a practical, benchtop alternative in the fingerprint region. More importantly, lasers offer an alternative to conventional broadband acquisition as well. Most notable is the capacity to acquire DFIR data, which can reduce acquisition time for an image by ~1000-fold. The focus on only a small part of the spectrum allows for potentially more detail-for example as seen in the newly developed two-dimensional (2D) IR microscopy (17) that offers spatially resolved measurements of two-dimensional infrared spectra. The diversity in instrumentation, using either continuous or DFIR approaches, now makes it possible to focus on either the spectral or spatial aspects of data as needed. This fundamentally alters the trade-off between scanning time, S/N, and the ability to extract information. It also opens up the possibility of designing data acquisition protocols to optimize relevant information extraction for a given problem. In this article, we illustrate these aspects of recent developments by providing an analysis of the trends and examples of the new possibilities.
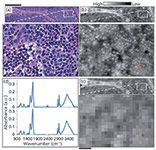
Spatial Collection Versus Discrete Frequency Interplay in IR Imaging
An emerging understanding of the fundamentals of IR image formation (19–21) has revealed the importance of correct spatial sampling to retrieve a fully resolved image, only limited by diffraction. Specifically, decreasing the pixel size to a value of ~1.1 µm (at 3950 cm-1) provides an optimal tradeoff between quality and speed. As opposed to the previously used 5.5–6.25 µm pixels, measurements at significantly higher densities are termed HD and were first shown using 12 defocused synchrotron beams to cover a 128×128 focal plane array (FPA) detector, reaching a pixel size of 0.54 µm (16). The use of a brighter synchrotron source was crucial as it allowed light to be better used with a high numerical aperture (NA) lens and allowed the acquisition of high S/N data in a time comparable to laboratory instruments. The smaller pixels imply a reduction in intensity compared to low NA–low magnification optics that can be mitigated by increasing the detector integration time, by better optical design, and by the use of mathematical noise rejection post-acquisition in laboratory HD systems. There are two major configurations at present-a thermal source (21) in a benchtop Fourier transform infrared (FT-IR) spectrometer where the sampling objective is either a 74× (NA = 0.65) or a 15× objective (NA = 0.65) with an additional 5× magnification optic just before the FPA detector (18,22,23). The combination of better optical design (22,23) and the use of numerical methods for noise rejection has now allowed both HD and high quality data in a reasonable amount of time. An example is shown in Figure 1, in which HD imaging of lymph nodes is shown to provide high quality histopathologic information that provides morphologic detail close to that obtained by conventional optical microscopy.
An alternative to speed up the acquisition time is to measure only frequencies of highest importance in the form of DFIR. This has initially been achieved by the use of spectral filters in a nonimaging setting (24). As opposed to the initial attempts to use filters for DFIR imaging, the use of QCL sources enables practical DFIR imaging because of the high brightness of the source (25–27). Most IR microscopes feature FT-IR spectrometers that include a broadband source and an interferometer. In contrast, the emerging QCL setup illuminates the sample using a single narrow spectral band at any given time. Commercial tunable QCL systems are typically a combination of up to four individual laser modules, each covering ~200 cm-1 of spectral bandwidth with a typical spectral step of 1 cm-1 and linewidth of 0.1 cm-1, allowing access to the full fingerprint region (26,28). QCL imaging studies have been performed using both a microbolometer (29) and a mapping approach, scanning every pixel point by point with a single-element mercury–cadmium–telluride (MCT) detector (25). A much higher imaging speed can be obtained using a wide-field approach, in which the beam is defocused to cover a large area of the sample that can be imaged with a multielement FPA (26,27) detector. In such setups the time gain per important frequency as compared to FT-IR can reach three orders of magnitude (27). Such systems are now commercially available (28). Additionally, the use of a large-format room-temperature microbolometer FPA is enabled by the high power of a QCL source sufficient to overcome its poor noise characteristics (30). This detector technology offers not only a cheaper alternative to MCT but also alleviates the need for liquid nitrogen cooling. Although smaller in format, MCT FPAs are lower in noise and have significantly faster readouts, allowing for significantly higher frame rates (submillisecond compared to ~10 ms for bolometers) (27). The faster speed and lower noise of this smaller format offsets the multichannel advantage of large bolometers with the tradeoff of increased cost and operating complexity.
Although comparison metrics can be applied to compare across multiple modalities, we emphasize that there is no straightforward way to compare these systems. Unlike the recent past, where use of an interferometer and high performance detector were essential elements of any IR imaging strategy, the diversity of instrumentation from choices of source, detector, and discrete frequency (DF)–Fourier transform (FT) approaches now means that instruments can be built for purpose more readily than ever before. We believe each type of instrument is best suited to a specific use-the QCL system is best for fast scanning at high spatial quality but limited spectral range, the FPA FT-IR system is best for high HD image quality and full spectrum imaging, and detectors such as linear array or point systems offer the potential for collecting very high quality spectra at an intermediate imaging speed. In light of this diversity, it is instructive to theoretically compare the systems by normalizing performance as per the trading rules of IR imaging (31). Two systems can be compared by means of a pixel rate ratio (R) that is proportional to the number of pixels (n), the acquisition time (t), and the resulting S/N (32)
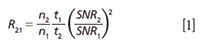
Since S/N has a time-squared dependence, we first seek to normalize the rate for equivalent S/N. The time to scan (t) is the next parameter and finally the number of pixels scanned in that time (n) is noted. At present, QCL microscopes provide the option of fast image scans at rates competitive with current FT-IR instruments. Often, the S/N is lower but the ability to scan only a few frequencies to provide informative images can make the information extraction more than 100-fold faster (33). One benefit from imaging single wavelengths at a time is the ability to use more optimized, refractive objectives because the focus can readily be adjusted for each wavelength of interest. It must also be realized that full maturity of laser-based instrumentation would require the development of sources with sufficient spectral coverage for evaluating the entire vibrational spectrum-current state-of-the-art equipment created in a laboratory covers the range from 1904 to 776 cm-1 using four QCL chips or sources (27), for example. Hence, we emphasize that the optimal use of QCL-based microscopes lies in their ability to make small sets of spectral measurements in the fingerprint region and that they are complementary, rather than competitive, with FT-IR imaging at this juncture.
The Effects of Sampling Geometry and Sample Requirements: An Enduring Challenge
Four main sampling geometries are typically used: transmission, transflection, reflection, and attenuated total reflection (ATR). Pure reflection measurements are limited to polished samples with a reflective surface and yield essentially measurements of the real part of the refractive index because of the low influence of absorption. Transmission and transflection cover the majority of experiments, and ATR is usually used in special cases of either thick or highly reflective samples (34). The former two require the light to pass through the thin specimen, and transflection's double pathlength through the sample exacerbates the pathlength requirement. Biological tissues limit the thickness to 10–20 µm, although recent demonstrations with QCLs suggest this may be extended to 50 µm or more. The ATR approach is free of this requirement because of the nature of the evanescent wave; however, there is another requirement that the ATR crystal must have a higher refractive index than the specimen (35). For each of these modalities, there is a growing understanding of optical effects and artifacts, and how these can be corrected to produce more accurate absorbance spectra. Scattering plays the major source of spectral distortion in both transmission and transflection modes and is quite well understood (19,20,36,37). In the transflection mode, attention has been brought (38,39) to the electric field standing wave (EFSW) effect, which is a consequence of light interference between the incoming and reflecting beams leading to unequal electric field intensities across different wavelengths of light within a sample. This introduces a peak ratio dependence on sample thickness, which is not a desirable feature in chemical imaging. However, a number of other works discussed the degree to which this effect degrades diagnostic potential both theoretically (40,41) and experimentally on cells (42) and on tissues (43,44)-for most of the tested systems it did not alter the analytical capabilities. The physical origins and dependence on sample properties (size, shape, refractive index) are known for the majority of systems, but the inverse problem-that is, to predict those properties from the scattering–absorption spectrum-remains to be rigorously solved. There are two existing algorithms that offer some degree of model-based correction: resonant Mie extended multiplicative scattering correction (RMie-EMSC) (45) and phase correction (46). There is an ongoing discussion about their applicability in various scenarios and they have not become a standard method of preprocessing FT-IR spectra. In many systems, the combination of second derivatives and normalization minimizes the contribution from optical effects.
ATR imaging typically minimizes scattering contributions because the sub-wavelength interaction of the evanescent wave with the sample (especially for high index substrate materials) minimizes scattering contributions. This dependence of the depth of penetration on the wavelength of light may cause problems for uneven sample volumes across the spectrum and the precision in spectral localization may be affected by the Goose-Hanchen shift (47).
The most recent advancements in sampling for IR imaging are developments toward IR spectroscopic tomography. Thanks to the high brilliance of a synchrotron source, three-dimensional (3D) images of various materials were obtained in the first reported IR tomography experiment (48) and were later supplemented with thermal source tomographic imaging of a single plant cell (49). Variable angle germanium ATR imaging was implemented into an IR microscope for depth profiling of polymers (50) and provides a different avenue to 3D information.
Also, ATR imaging effectively increases magnification; depending on the geometry and material of the crystal, the additional magnification introduced is typically in the 4–5× range. Thus, there is a higher inherent resolution advantage, which appears to match that of the HD optics case for a pixel size of ~1–1.5 mm with a "conventional" 15× objective (47) and 0.22–0.275 mm with the additional 5× optics before the detector (22). An innovative implementation of CaF2 pseudo-hemisphere to transmission measurements of flow cells (51) provides several advantages of the increased magnification. In addition to reducing chromatic aberration, the introduction of the pseudo-hemisphere also forms a solid lens, effectively increasing the NA by a factor equal to the refractive index of the material-that is, 1.4 for CaF2. The index matching with a sample also results in reduced scattering. Further improvement of this approach in the form of large-scale mapping with the pseudo-hemisphere attached to the objective has recently been achieved (52), paving the way for larger tissue specimen imaging.
Applications Illustrating the HD and DF Trends
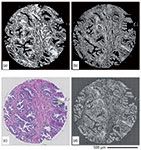
Because HD FT-IR imaging is a relatively new development, only a handful of applications have been demonstrated. The increase in image quality is desirable for tissue imaging (22), single-cell imaging (23), and classification purposes; however, there is a concern that additional variability, possibly originating from subcellular contributions in the case of tissues, may hinder the potential of the technique to create robust models of the tissue structure. Such behavior was observed for lymphatic tissue (18), where unsupervised hierarchical cluster analysis (HCA) was influenced by nontissue-specific signals when a pixel size of 1.1 mm was used. When at least four (2×2) pixels were averaged, HCA was driven again by tissue type variability. This problem has been overcome with a supervised random forest classification algorithm. An eight-class model was successfully created, being able to distinguish naive and memory B cells, T cells, light-zone-activated B cells, and dark-zone-activated B cells. Recent preliminary results also show that prostate tissue classification does not suffer a decrease in accuracy by using HD optics (53). Although these developments are relatively less demanding for spectral consistency, further tests of the HD approach will become more apparent when applications push the diagnostic limits in analyses such as the prediction of prostate cancer recurrence (54) outperforming other state-of-the-art methods or in more detailed cancer classification providing accurate subtyping of lung cancer (55). An example of this progress lies in the use of HD optics allowing the study of colon tissue in much higher detail, allowing discrimination of goblet cells along with their mucin secretions (56). Progress in breast tissue analysis using HD imaging has also been reported (57), followed by the creation of a tissue structure classification (58). In other important applications, HD imaging has also proven to be useful for identifying parasites in single erythrocytes (59) by increasing spatial unmixing of the parasite signal, and in studying tendon damage using linearly polarized light (60).
We envisage the use of both technologies in discovery or development of protocols (FT-IR) and tools for rapid translation to routine use (DFIR via QCL microscopy). An example of this approach has recently been demonstrated (33) in the identification of fibrosis within anonymized cardiovascular samples from human patients. Images obtained with a DFIR instrument were classified accurately by an algorithm trained on FT-IR data. A second algorithm trained and calibrated on DFIR data showed similar accuracy. Furthermore, demonstrating the potential of the DFIR approach, a single-band-based detection also correlated well with instances of fibrosis as identified by a pathologist. The speed of DFIR instruments in providing histologic images was at least 100 times higher than the fastest FT-IR imaging. QCL-based DFIR is being used in rapid tissue imaging of breast tissue (27,61), classification of prostate cancer cases (62), imaging of colon tissue (30,63) structure, and general imaging of brain (64), cardiac tissue (33), and lungs (65). Another branch of application comes in the form of biofluids (66), either serum (28,67) or protein solutions (68,69). Finally, an initial surface-enhanced infrared absorption (SEIRA) experiment with QCL has been reported (70) along with an application of live-organism monitoring (71), which are novel directions for IR imaging.
Raman Spectroscopic Imaging
Recent Advances in Raman Microscopy
The field of Raman microscopy continues to rapidly grow (72), especially in imaging. Several advances in instrumentation for spontaneous Raman microscopy have occurred within the past few years, including a better understanding of the use of confocal depth profiling (73), temporal filtering for fluorescence rejection (74), spectral filtering for compressive sensing (75), the incorporation of cell-isolation mechanisms (76), and numerous examples of novel surface-enhanced substrates (77,78). On the nonlinear Raman side, however, there is tremendous excitement and a growing number of studies using coherent Raman scattering (CRS) imaging methods. In particular, stimulated Raman scattering (SRS) microscopy has brought forth useful new instrumentation configurations and image acquisition strategies. Although it is a nascent technology with some technical hurdles yet to be overcome, SRS has enabled impressive biological investigations that use only a handful of Raman frequencies, which lends itself naturally to a discrete-frequency approach. Several examples include monitoring fat uptake in individual cells (79) and microscopic organisms (80); imaging DNA, protein, and lipid components in live cells (81); detecting tumors in brain tissue (82); and deep tissue imaging in a living patient (83).
Discrete-Frequency Strategies in Nonlinear Raman
Nonlinear Raman microscopy is now a well-established field of techniques (6,84,85), with a main draw being a faster imaging alternative to spontaneous Raman microscopy. Coherent anti-Stokes Raman (CARS) microscopy was demonstrated (86) early, but accelerated rapidly in its development with the advent of practical designs, lasers and computational tools, both software and hardware over the past two decades (87–91). Today, fully broadband implementations (92) as well as single-frequency approaches (88) exist. SRS microscopy was first demonstrated more recently (93) and now also features both broadband and single-frequency implementations. There have been numerous excellent studies in these fields that use information gained from single Raman bands or otherwise limited spectral information; as such, discrete-frequency approaches have been actively applied in this field for some time.
Although the two techniques are similar in implementation, they are not redundant technologies. CARS spectra feature a nonresonant background that distorts band shapes and results in significant deviations from corresponding spontaneous Raman spectra. Considerable work has been dedicated to strategies for correcting or eliminating its presence, including time-resolved measurements (94), polarization and phase control (95,96), and interferometric detection methods (97,98), and in fact it does contain additional information that may be used to better understand samples. However, this background represents a confounding contribution for DF imaging because contributions to the vibrational mode cannot easily be assigned independent of the knowledge of the background. To apply a correction or resolve the entire band shape, multiple measurements must be made, precluding truly discrete-frequency approaches with CARS. SRS does not have this nonresonant component, so there is greater spectral certainty when measuring one single frequency of interest.
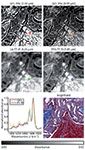
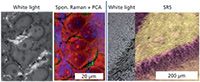
The close correspondence to spontaneous spectra plus the linear relationship between concentration and measured signal means that SRS is uniquely suited to truly discrete-frequency approaches analogous to the DFIR examples discussed previously. An interesting comparison can be seen between SRS and QCL-IR imaging in Figure 3. It can be seen that both technologies produce an excellent fidelity and detailed view of biological tissue. Although the signal-to-noise ratio of the QCL system is much higher, providing for better contrast, the resolution of the SRS system is superior, providing for more spatial detail. In both cases, the image of this biological tissue does not require the use of dyes or stains and provides sufficient quality to perform studies. Just as HD-IR advantages are becoming clear in emerging studies, the near future will likely provide a number of applications of detailed histopathology with SRS imaging. Given the two-photon origin of the signal in SRS, the optical sectioning of tissue in 3D will always be superior and provide detail in depth scanning applications, which are ultimately limited by scattering-induced loss of signal. Because this technology holds the potential to continue driving discrete-frequency approaches in Raman imaging, it is useful to highlight the different ways in which narrow-band or limited bandwidth SRS has been implemented.
The first implementation of SRS for microscopy was broadband in nature (93), but the first implementation that was appropriate for biological microscopy (lower power, faster speed) was single-frequency (79). Using two picosecond beams with a narrow spectral bandwidth (~3 cm-1) provides several advantages, including good spectral resolution and certainty of which Raman mode was being sampled. One significant drawback to such an approach is that accessing multiple Raman bands requires a series of several distinct single-band images, tuning the light source to match the Raman shift of interest between each acquisition. Two main disadvantages arise from this. First, while individual images can be acquired very quickly, a series of images can be slow and tedious, greatly mitigating the speed advantage afforded by the technique. Second, there will always be some uncertainty in spatial colocalization across a series of many images. This becomes even more complicated when imaging a system that evolves in time, and limits the amount of useful spectral or spatial data that can be acquired.
Since then, several strategies have been developed to multiplex or otherwise facilitate the acquisition of multiple frequencies without sacrificing imaging speed (99,100). The challenges associated with multifrequency measurements are different than those for QCL-IR instruments; optical parametric oscillators (OPOs), the most common choice for a tunable coherent Raman scattering (CRS) source, often involve a temperature-changing element, the adjustment of which represents the slowest step in a SRS experiment. Whereas the full tunable range of a commercial QCL source can be frequency-swept in a fraction of a second, CRS sources are often limited in this respect. One straightforward way of multiplexing Raman frequencies in an SRS experiment is to use multiple tunable beams simultaneously. If each of these is intensity modulated at a different frequency, a single-element detector can still be used to measure the Raman generation of each pair of colors (100). Of course, this strategy is ultimately limited by cost (both OPOs and lasers of sufficient quality to drive them often cost as much as entire commercial instruments) and by the total amount of optical power that can be injected into the specimen. As such, this strategy is not infinitely extendible. As opposed to multiplexing, another strategy for increasing the speed at which spectral information is acquired has been to develop OPO components that allow for rapid tuning over small spectral ranges (101). At any given temperature setting, an OPO is capable of producing light from a narrow range of wavelengths (in the near infrared [NIR] this is often on the order of 10–15 nm). Quickly sweeping over this range can be achieved by specialized motors that control an optical filter. Therefore, much like with QCL operation, a spectral image over a given field of view can be acquired in a reasonable amount of time. Although this spectral range is limited, it can allow researchers to acquire a spectral image without any movement of the stage or specimen.
A third type of approach uses "spectrally tailored" pulses with a width of ~200 cm-1 to detect one specific compound of interest at a time (80). In any sort of organic setting, most constituents within a specimen will have signatures in the "C-H stretching" region. While these bands may overlap, there are distinct contributions from each constituent, and if their spectra are known in advance it is possible to calculate a pair of spectral components that are unique to a given compound. By shaping a pulse to match this "spectrally orthogonal" component, it is possible to create a single image that highlights only that compound. This is not a truly broadband measurement because a single-element detector is used; rather it is a type of "spectrally compressive sensing" that is implemented in a manner similar to single-frequency measurements, retaining a rapid imaging speed.
As multiband approaches in CRS become increasingly feasible, new detector types will need to be developed to match. A single-element, nonintegrating analog detector has the desirable characteristics of fast dwell time, large dynamic range, and relatively straightforward implementation. For SRS, the Raman signal is often frequency-encoded and evaluated by a lock-in amplifier or other demodulation strategy (79). This instrumentation is bulky and expensive, and can only be practically multiplexed to a degree. New circuitry that aims to replace lock-in detection has been developed which is more compact and can be housed inside of a single photodetector element (102,103). The performance has been shown to exceed lock-in detection for some cases, and the practicality of such an approach indicates that it may enable and drive the development of complex multiband or broadband CRS measurement strategies.
Sampling Geometry and Sample Requirements
Unlike the four distinct sampling geometries used in IR microscopy, Raman and CRS illumination strategies can be boiled down to reflection (also referred to as backscatter or "epi") and transmission. Commercial spontaneous microscopes almost unanimously default to collecting backscattered Raman photons, because this approach is generally compatible with specimens of any thickness or with less-than-ideal optical transmission properties. Even so, many biological specimens are weakly scattering and absorbing in the near-infrared (700–1000 nm) region (104), and so a transmission geometry becomes a viable option for "thin" specimens on the order of 100 µm thick or less. For SRS, this can enable simpler optics and a greater ability to collect Raman signal, whereas CARS performed in an "epi" geometry may have reduced background contributions (87). In fact, CARS and SRS have often been constructed on platforms that allow "epi" and "trans" scatter of Raman photons to be collected simultaneously (79,88). Other novel geometries have been implemented for CRS, including endoscopy (105,106) and standoff detection (107), but these fall outside the scope of this review.
The choice of objective optics is important for best meeting the needs of a given specimen. Water and oil immersion objectives can be helpful because they enable tighter focusing and minimize the effects of interfaces or other refractive index changes that may distort images (73), especially when performing depth profiling. An oil condenser for transmission illumination can be especially helpful (79). Collecting as much light as possible maximizes throughput, so a high numerical aperture with a wide field of view is ideal. Another approach that has been used to maximize the collection of scattered signal for deep tissue imaging is to use a large-area donut-shaped photodiode that encircles the front aperture of the microscope objective (83). This approach has proven successful in performing in vivo deep-tissue imaging.
Nonlinear extensions of Raman microscopy also bring new technical challenges and unwanted optical effects. It has been well-established that CRS does not suffer from the presence of fluorescence, and although it lacks the nonresonant background of CARS, SRS is not without interfering optical phenomena. Cross-phase modulation (108–110) and thermal lensing, caused by intense electrical fields leading to frequency-modulated changes in refractive index, can obfuscate information collected from weakly Raman-scattering specimens (79) and can lead to aberrant imaging artifacts. Additionally, a spectral filter cannot be used to separate SRS signal from residual pump or probe, creating challenges for detection and dynamic range. The use of multiple excitation beams requires users to confront chromatic aberration in sample illumination. These concerns are exacerbated by broadband measurements and require careful consideration of how spatial overlap of multiple colors of light changes as a function of wavelength difference, specimen properties, imaging depth, and so forth. Together, these effects remain to be explored. Unlike IR imaging, where extensive theory on the interactions of samples with light, effects of microstructure, collection optics, and sampling have been well studied now, these studies are not extensive but are likely to emerge for SRS microscopy as well.
Applications Illustrating DF Trends from the Literature
In addition to the examples mentioned previously, there are numerous excellent studies in the literature that use CRS imaging at a handful of Raman shifts to conduct biological investigations. Using independent component (111) or singular value decomposition analysis (112), efforts have been made to identify and discriminate subcellular components in a more automated fashion. Beyond visualizing the distribution of tissue components, specific features of tissue have been investigated with CRS to elucidate a physiological or disease state. This includes the analysis of atherosclerotic plaques (113), the identification of squamous cell carcinoma (114), and the presence of liver damage (115). In vivo deep-tissue analysis has been demonstrated for both CARS (116) and SRS (83). Both of these studies demonstrate the ability to image liquids diffusing into tissue, enabled by rapid imaging speed and good depth profiling capabilities. The specificity of CRS imaging has also paved the way for the development of CRS-specific probes, including alkyne tags (117) and deuterium labeling for monitoring protein formation (118) and lipid membrane analysis (119) and gold SERS nanoparticles designed for investigating single-molecule signals (120). The ability to combine multiple linear and nonlinear imaging modalities onto a single platform allows for gaining insight about the properties of a specimen beyond the vibrational spectrum (121). Several excellent reviews and tutorials exist in the literature for those interested in applying CRS to their own studies (6,85,122). This is far from an exhaustive list of examples, but even from this sampling it is clear that CARS and SRS will continue to drive discrete-frequency approaches on the Raman side of vibrational chemical imaging.
Summary and Conclusions
With the advent of QCLs that are appropriate for spectroscopic infrared microscopy and the increasing popularity of nonlinear Raman imaging methods, discrete-frequency approaches to chemical imaging are proving to be viable methods of investigation. FT-IR and spontaneous Raman spectroscopic imaging methodologies, while proven and robust, undoubtedly result in the collection of some "unnecessary" spectral information; by eliminating this, great gains can be made in imaging speed, resolution, and time-resolved capabilities. It is the authors' opinion that this approach to microscopic investigation will become well-suited for a number of tasks and result in an important complementary technique to "full spectral" approaches. Ultimately, the decision about which technique to apply to a given sample or problem will depend on the particular set of spatial and spectral requirements. For low-component-number systems with large domain sizes, discrete-frequency approaches coupled to nondiffraction limited resolutions will enable rapid and high-throughput analysis. Multicomponent systems with small domain sizes will require significant spectral and spatial sampling and may be best suited by a traditional approach. Proper exploration of a given system, through spontaneous Raman or FT-IR imaging, may elucidate a limited number of spectral points necessary to acquire and enable discrete-frequency approaches for problem solving, allowing for efficiency via QCL, SRS, or CARS methods.
Acknowledgments
We would like to acknowledge generous support from the entities for resources that contributed toward or were featured in this review. We are grateful to Thermo Scientific for use of a Thermo Scientific DXR xi imaging microscope. We are thankful to Agilent Technology for equipment support. Thanks to the Martha Gillete Lab at the University of Illinois at Urbana-Champaign for the rat brain specimen. TPW acknowledges support through the "Mobility Plus" program from the Polish Ministry of Science and Higher Education. We gratefully acknowledge funding from the National Institutes of Health via grants R01EB009745, R21CA190120, and U01MH109062.
References:
(1) S. Lohumi, S. Lee, H. Lee, and B.-K. Cho, Trends Food Sci. Technol. 46(1), 85–98. 10.1016/j.tifs.2015.08.003 (2015).
(2) R. Gautam et al., Curr. Sci. 108(3), 341–356 (2015).
(3) A.A. Gowen, Y. Feng, E. Gaston, and V. Valdramidis, Talanta 137, 43–54, 10.1016/j.talanta.2015.01.012 (2015).
(4) S. Mukherjee and A. Gowen, Anal. Chim. Acta. 895, 12–34, 10.1016/j.aca.2015.09.006 (2015).
(5) A.A. Bunaciu, H.Y. Aboul-Enein, and V.D. Hoang, TrAC–Trends Anal. Chem. 69, 14–22, 10.1016/j.trac.2015.02.006 (2015).
(6) J.-X. Cheng and X.S. Xie, Science 350(6264), aaa8870. 10.1126/science.aaa8870 (2015).
(7) H.J. Humecki, Practical Guide to Infrared Microspectroscopy (CRC Press Book, Marcel Dekker Inc., New York, New York,1995).
(8) E.R. Blout, G.R. Bird, and D.S. Grey, J. Opt. Soc. Am. 40(5), 304–313 (1950).
(9) P.J. Treado, I.W. Levin, and E.N. Lewis, Appl. Spectrosc. 48(5), 607–615, 10.1366/0003702944924899 (1994).
(10) P. Colarusso, L.H. Kidder, I.W. Levin, J.C. Fraser, J.F. Arens, and E.N. Lewis, Appl. Spectrosc. 52(3), 106A–120A (1998).
(11) M.D. Schaeberle, H.R. Morris, J.F.T. II, and P.J. Treado, Anal. Chem. 71(5), 175–181 (1999).
(12) H. Tu and S.A. Boppart, J. Biophotonics. 7(1-2), 9–22, 10.1002/jbio.201300031 (2014).
(13) R. Bhargava, S. Wang, and J.L. Koenig, Adv. Polym. Sci. 163, 137–191, 10.1007/b11052 (2003).
(14) R. Bhargava, D.C. Fernandez, S.M. Hewitt, and I.W. Levin, Biochim. Biophys. Acta. 1758(7), 830–845, 10.1016/j.bbamem.2006.05.007 (2006).
(15) A.K. Kodali, M. Schulmerich, J. Ip, G. Yen, B.T. Cunningham, and R. Bhargava, Anal. Chem. 82(13), 5697–5706 (2010).
(16) M.J. Nasse et al., Nat. Methods 8(5), 413–418, 10.1038/NMETH.1585 (2011).
(17) C.R. Baiz, D. Schach, and A. Tokmakoff, Opt. Express. 22(15), 18724–18735, 10.1364/OE.22.018724 (2014).
(18) L.S. Leslie, T.P. Wrobel, D. Mayerich, S. Bindra, R. Emmadi, and R. Bhargava, PLoS One 10, e0127238, 10.1371/journal.pone.0127238 (2015).
(19) B.J. Davis, P.S. Carney, and R. Bhargava, Anal. Chem. 82(9), 3474–3486, 10.1021/ac902068e (2010).
(20) B.J. Davis, P.S. Carney, and R. Bhargava, Anal. Chem. 82(9): 3487–3499, 10.1021/ac902067p.10.1021/ac902068e (2010).
(21) R.K. Reddy, M.J. Walsh, M. V Schulmerich, P.S. Carney, and R. Bhargava, Appl. Spectrosc. 67(1), 93–105, 10.1366/11-06568 (2013).
(22) C.R. Findlay et al., Analyst 140(7), 2493–2503, 10.1039/C4AN01982B (2015).
(23) C. Hughes et al., Analyst 140, 2080–2085, 10.1039/C4AN02053G (2015).
(24) D. Rossberg, Sensors and Actuators A: Physical. 47(1–3), 413–416, 10.1016/0924-4247(94)00932-8 )1995).
(25) M.R. Kole, R.K. Reddy, M. V Schulmerich, M.K. Gelber, and R. Bhargava, Anal. Chem. 84(23), 10366–10372, 10.1021/ac302513f (2012).
(26) K. Yeh, M. Schulmerich, and R. Bhargava, in Proceedings of the SPIE, M.A. Druy and R.A. Crocombe, Eds., 8726(217), 87260E, 10.1117/12.2015984 (2013).
(27) K. Yeh, S. Kenkel, J.-N. Liu, and R. Bhargava, Anal. Chem. 87(1), 485–493, 10.1021/ac5027513, http://pubs.acs.org/doi/full/10.1021/ac5027513 (2015).
(28) G. Clemens, B. Bird, M. Weida, J. Rowlette, and M.J. Baker, Spectrosc. Eur. 26(4), 14–19 (2014).
(29) M.C. Phillips and N. Ho, Opt. Express. 16(3), 1836–1845, 10.1364/OE.16.001836 (2008).
(30) N. Kröger et al., J. Biomed. Opt. 19, 111607-1–111607-6, 10.1117/1.JBO.19.11.111607 (2014).
(31) R. Bhargava and I.W. Levin, Anal. Chem. 73(21), 5157–5167 (2001).
(32) R. Bhargava and I.W. Levin, Spectrochemical Analysis Using Infrared Multichannel Detectors (Blackwell Publishing Ltd., Oxford, UK, 2008).
(33) S. Tiwari et al., Anal. Chem. 2016, In review.
(34) T.P. Wrobel et al., Analyst 137(18), 4135–4139, 10.1039/c2an35331h (2012).
(35) K.M. Marzec et al., J. Biophotonics. 7(9), 744–756, 10.1002/jbio.201400014 (2014).
(36) P. Bassan et al., Analyst 134(6), 1171–1175, 10.1039/b821349f (2009).
(37) B.J. Davis, P.S. Carney, and R. Bhargava, Anal. Chem. 83(2), 525–532, 10.1021/ac102239b (2011).
(38) J. Filik, M.D. Frogley, J.K. Pijanka, K. Wehbe, and G. Cinque, Analyst 137(4), 853–861, 10.1039/c2an15995c (2012).
(39) P. Bassan et al., Analyst 138, 144–157, 10.1039/c2an36090j (2012).
(40) T.P. Wrobel, B. Wajnchold, H.J. Byrne, and M. Baranska, Vib. Spectrosc. 69, 84–92, 10.1016/j.vibspec.2013.09.008 (2013).
(41) T.P. Wrobel, B. Wajnchold, H.J. Byrne, and M. Baranska, Vib. Spectrosc. 71, 115–117, 10.1016/j.vibspec.2014.01.006 (2014).
(42) J. Cao et al., Analyst 138(14), 4147–4160, 10.1039/c3an00321c 92013. (2013).
(43) D. Perez-Guaita, P. Heraud, K.M. Marzec, M. de la Guardia, M. Kiupel, and B.R. Wood, Analyst 140(7), 2376–2382, 10.1039/C4AN02034K (2015).
(44) K. Kochan et al., Analyst 140(7), 2402–2411, 10.1039/C4AN01901F (2015).
(45) P. Bassan et al., J. Biophotonics. 3(8-9), 609–620, 10.1002/jbio.201000036 (2010).
(46) M. MiljkoviÄ, B. Bird, and M. Diem, Analyst 137(17), 3954–3964, 10.1039/c2an35582e (2012).
(47) S.G. Kazarian and K.L.A. Chan, Analyst 138(7), 1940–1951, 10.1039/c3an36865c (2013).
(48) M.C. Martin et al., Nat. Methods 10(9), 861–864, 10.1038/nmeth.2596 (2013).
(49) L. Quaroni, M. Obst, M. Nowak, and F. Zobi, Angew. Chemie Int. Ed. 54(1), 318–322, 10.1002/anie.201407728 (2015).
(50) T.P. Wrobel, A. Vichi, M. Baranska, and G. Kazarian, Appl. Spectrosc. 69(10), 8–13, 10.1366/15-07963 (2015).
(51) K.L.A. Chan and S.G. Kazarian, Anal. Chem. 85(2), 1029–1036, 10.1021/ac302846d (2013).
(52) J.A. Kimber, L. Foreman, B. Turner, P. Rich, and S.G. Kazarian, Faraday Discuss. 10.1039/c5fd00158g (2016).
(53) T.P. Wrobel, J.T. Kwak, A. Kadjacsy-balla, and R. Bhargava, Proc. SPIE. 9791, 97911D, DOI: 10.1117/12.2217341 (2016).
(54) J.T. Kwak, A. Kajdacsy-Balla, V. Macias, M. Walsh, S. Sinha, and R. Bhargava, Sci. Rep. 5, 8758, 10.1038/srep08758 (2015).
(55) F. Großerueschkamp et al., Analyst 140(140), 2114–2120 (2015).
(56) J. Nallala, G.R. Lloyd, N. Shepherd, and N. Stone, Analyst 141(2), 630–639, 10.1039/C5AN01871D (2016).
(57) L.S. Leslie, A. Kadjacsy-Balla, and R. Bhargava, Prog. Biomed. Opt. Imaging - Proc. SPIE. 9420, 94200I, 10.1117/12.2082461 (2015).
(58) S. Mittal, T.P. Wrobel, L.S. Leslie, and A. Kadjacsy-balla, Prog. Biomed. Opt. Imaging - Proc. SPIE. 9420, 10.1117/12.2217358 (2016).
(59) D. Perez-Guaita et al., Faraday Discuss. 10.1039/C5FD00181A (2015).
(60) R.A. Wiens et al., Faraday Discuss. 10.1039/C5FD00168D (2016).
(61) P. Bassan, M.J. Weida, J. Rowlette, and P. Gardner, Analyst 139, 3856–3859, 10.1039/c4an00638k (2014).
(62) M.J. Pilling, P. Gardner, A. Henderson, M.D. Brown, B. Bird, and N.W. Clarke, Faraday Discuss. 10.1039/C5FD00176E (2016).
(63) B. Bird and M.J. Baker, Trends Biotechnol. 33(10), 557–558, 10.1016/j.tibtech.2015.07.003 (2015).
(64) A. Mertiri et al., “Label Free Mid-IR Photothermal Imaging of Bird Brain with Quantum Cascade Laser,” presented at Cleo 2014, San Jose, California, 2014, 10.1364/CLEO_AT.2014.AF1B.4.
(65) K. Yeh, M. Schulmerich, and R. Bhargava, in Proc. SPIE. M.A. Druy and R.A. Crocombe, Eds. 8726(217), 10.1117/12.2015984 (2013).
(66) C. Hughes et al., Sci. Rep. 6(February), 20173, 10.1038/srep20173 (2016).
(67) A. Mukherjee, Q. Bylund, M. Prasanna, Y. Margalit, and T. Tihan, J. Biomed. Opt. 18(3), 036011-1–036011-4, 10.1117/1.JBO.18.3.036011 (2013).
(68) K. Haase, N. Kröger-Lui, A. Pucci, A. Schönhals, and W. Petrich, Faraday Discuss. 10.1039/C5FD00177C (2015).
(69) M.R. Alcaráz et al., Anal. Chem. 87(13), 6980–6987, 10.1021/acs.analchem.5b01738 (2015).
(70) A. Hasenkampf, N. Kröger, A. Schönhals, W. Petrich, and A. Pucci, Opt. Express. 23(5), 5670–5680, 10.1364/OE.23.005670 (2015).
(71) K. Haase, N. Kröger-Lui, A. Pucci, A. Schönhals, and W. Petrich, J. Biophotonics. 9(1–2), 61–66, 10.1002/jbio.201500264 (2016).
(72) L.A. Nafie, J. Raman Spectrosc. 46(12), 1173–1190 (2015).
(73) N. Everall, J. Raman Spectrosc. 45(1), 133–138, 10.1002/jrs.4430 (2014).
(74) A. Ehn, M. Levenius, M. Jonsson, M. Aldén, and J. Bood, J. Raman Spectrosc. 44(4), 622–629, 10.1002/jrs.4235 (2013).
(75) B.M. Davis, A.J. Hemphill, D. Cebeci Maltas, M.A. Zipper, P. Wang, and D. Ben-Amotz, Anal. Chem. 83(13), 5086–5092, 10.1021/ac103259v (2011).
(76) Y. Wang et al., Anal. Chem. 85(22), 10697–10701, 10.1021/ac403107p (2013).
(77) B. Sharma, R.R. Frontiera, A.-I. Henry, E. Ringe, and R.P. Van Duyne, Mater. Today. 15(1–2), 16–25, 10.1016/S1369-7021(12)70017-2 (2012).
(78) W. Xu, N. Mao, and J. Zhang, Small 9(8), 1206–1224, 10.1002/smll.201203097 (2013).
(79) C.W. Freudiger et al., Science 322(5909), 1857–1861, 10.1126/science.1165758 (2008).
(80) C.W. Freudiger, W. Min, G.R. Holtom, B. Xu, M. Dantus, and X.S. Xie, Nat. Photonics. 5, 103–109, 10.1038/nphoton.2010.294 (2011).
(81) F. Lu et al., Proc. Natl. Acad. Sci. 112(37), 11624–11629 (2015).
(82) M. Ji et al., Sci. Transl. Med. 5(201), 201ra119 1–10, 10.1126/scitranslmed.3005954 (2013).
(83) B.G. Saar, C.W. Freudiger, J. Reichman, C. Stanley, G.R. Holtom, and X.S. Xie, Science 330(December), 1368–1370 (2010).
(84) Y. Yu, P. V. Ramachandran, and M.C. Wang, Biochim. Biophys. Acta. 1841, 1120–1129, 10.1016/j.bbalip.2014.02.003 (2014).
(85) W. Min, C.W. Freudiger, S. Lu, and X.S. Xie, Annu. Rev. Phys. Chem. 62, 507–530, 10.1146/annurev.physchem.012809.103512 (2011).
(86) M.D. Duncan, J. Reintjes, and T.J. Manuccia, Opt. Lett. 7(8), 350–352, 10.1364/OL.7.000350 (1982).
(87) E.O. Potma and X.S. Xie, Opt. Photonics News. 5(11), 40–45 (2004).
(88) J.X. Cheng and X.S. Xie, J. Phys. Chem. B. 108, 827–840, 10.1039/c3cc45319g (2004).
(89) J.-X. Cheng, Y.K. Jia, G. Zheng, and X.S. Xie, Biophys. J. 83, 502–509, 10.1016/S0006-3495(02)75186-2 (2002).
(90) C.L. Evans and X.S. Xie, Annu. Rev. Anal. Chem. 1, 883–909, 10.1146/annurev.anchem.1.031207.112754 (2008).
(91) J.X. Cheng, Appl. Spectrosc. 61(9), 197A–208A, 10.1002/cphc.200700202 (2007).
(92) T.W. Kee and M.T. Cicerone, Opt. Lett. 29(23), 2701–2703, 10.1364/OL.29.002701 (2004).
(93) E. Ploetz, S. Laimgruber, S. Berner, W. Zinth, and P. Gilch, Appl. Phys. B Lasers Opt. 87(3), 389–393, 10.1007/s00340-007-2630-x (2007).
(94) A. Volkmer, L.D. Book, and X.S. Xie, Appl. Phys. Lett. 80(9), 1505–1507, 10.1063/1.1456262 (2002).
(95) J.X. Cheng, L.D. Book, and X.S. Xie, Opt. Lett. 26(17), 1341–1343, 10.1364/OL.26.001341 (2001).
(96) D. Oron, N. Dudovich, and Y. Silberberg, Phys. Rev. Lett. 90(21), 213902, 10.1103/PhysRevLett.90.213902 (2003).
(97) C.L. Evans, E.O. Potma, and X.S. Xie, Opt. Lett. 29(24), 2923–2925, 10.1364/OL.29.002923 (2004).
(98) D.L. Marks and S.A. Boppart, Phys. Rev. Lett. 92(12), 123905, 10.1103/PhysRevLett.92.123905 (2004).
(99) B.G. Saar et al., Angew. Chemie Int. Ed. 49, 5476–5479, 10.1002/anie.201000900 (2010).
(100) D. Fu et al., J. Am. Chem. Soc. 134, 3623–3626, 10.1021/ja210081h (2012).
(101) L. Kong, M. Ji, G.R. Holtom, D. Fu, C.W. Freudiger, and X.S. Xie, Opt. Lett. 38(2), 145–147, 10.1364/OL.38.000145 (2013).
(102) M.N. Slipchenko, R. a. Oglesbee, D. Zhang, W. Wu, and J.X. Cheng, J. Biophotonics. 5(10), 801–807, 10.1002/jbio.201200005 (2012).
(103) C.-S. Liao et al., Light Sci. Appl. 4, e265, 10.1038/lsa.2015.38 (2015).
(104) A.M. Smith, M.C. Mancini, and S. Nie, Nat. Nanotechnol. 4, 710–711, 10.1038/nnano.2009.326 (2009).
(105) B.G. Saar, R.S. Johnston, C.W. Freudiger, X.S. Xie, and E.J. Seibel, Opt. Lett. 36(13), 2396–2398, 10.1364/OL.36.002396 (2011).
(106) F. Légaré, C.L. Evans, F. Ganikhanov, and X.S. Xie, Opt. Express. 14(10), 4427–4432, 10.1364/OE.14.004427 (2006).
(107) H. Li, D.A. Harris, B. Xu, P.J. Wrzesinski, V. V Lozovoy, and M. Dantus, Appl. Opt. 48(4), B17–B22, 10.1364/AO.48.000B17 (2009).
(108) Q. Hong, J. Durrant, G. Hastings, G. Porter, and D.R. Klug, Chem. Phys. Lett. 202, 183–185, 10.1016/0009-2614(93)85262-M (1993).
(109) K. Ekvall et al., J. Appl. Phys. 87(5), 2340–2352, 10.1063/1.372185 (2000).
(110) R.R. Alfano, P.L. Baldeck, P.P. Ho, and G.P. Agrawal, J. Opt. Soc. Am. B. 6(4), 824–829, 10.1364/JOSAB.6.000824 (1989).
(111) Y. Ozeki et al., Nat. Photonics. 6, 845–851, 10.1038/NPHOTON.2012.263 (2012).
(112) A. Khmaladze et al., Appl. Spectrosc. 68(10), 1116–1122, 10.1366/13-07183 (2014).
(113) J.L. Suhalim et al., Biophys. J. Biophysical Society 102(8), 1988–1995. 10.1016/j.bpj.2012.03.016 (2012).
(114) R. Mittal et al., Lasers Surg. Med. 45, 496–502, 10.1002/lsm.22168 (2013).
(115) S. Satoh et al., Pathol. Int. 64, 518–526, 10.1111/pin.12206 (2014).
(116) C.L. Evans, E.O. Potma, M. Puoris’haag, D. Côté, C.P. Lin, and X.S. Xie, Proc. Natl. Acad. Sci. U.S.A. 102(46), 16807–16812, 10.1073/pnas.0508282102 (2005).
(117) L. Wei et al., Nat. Methods. 11(4), 410–412, 10.1038/nmeth.2878 (2014).
(118) L. Wei, Y. Yu, Y. Shen, M. Wang, and W. Min, Proc. Natl. Acad. Sci. 110, 11226–11231, 10.1073/pnas.1303768110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1303768110 (2013).
(119) A. Alfonso-García, S.G. Pfisterer, H. Riezman, E. Ikonen, and E.O. Potma, J. Biomed. Opt. 21(6), 61003, 10.1117/1.JBO.21.6.061003 (2016).
(120) S. Yampolsky et al., Nat. Photonics. 8, 650–656, 10.1038/nphoton.2014.143 (2014).
(121) S. Yue, M. Slipchenko, and J. Cheng, Laser Photonics Rev. 5(4), 496–512, 10.1002/lpor.201000027 (2011).
(122) A. Alfonso-García, R. Mittal, E.S. Lee, and E.O. Potma, J. Biomed. Opt. 19(7), 71407, 10.1117/1.JBO.19.7.071407 (2014).
Tomasz P. Wrobel is with the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign in Urbana, Illinois. Matthew R. Kole is with the Beckman Institute for Advanced Science and Technology and the Department of Bioengineering at the University of Illinois at Urbana-Champaign. Rohit Bhargava is with the Beckman Institute for Advanced Science and Technology, the Department of Bioengineering, and the Departments of Chemical & Biomolecular Engineering, Electrical & Computer Engineering, Mechanical Science & Engineering and Chemistry at the University of Illinois at Urbana-Champaign. Direct correspondence to: rxb@illinois.edu

Trends in Infrared Spectroscopic Imaging
September 13th 2013An interview with Rohit Bhargava, winner of the 2013 Craver Award. This interview is part of the 2013 podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2013, the federation?s North American conference.
Staying Updated with Spectroscopic Techniques: How Lead Investigators Adapt to a Changing Industry
June 6th 2024Spectroscopy is at the forefront of many changes happening across many industries. Here, three lead investigators comment on how they stay updated with the latest innovations and developments.
Advancing Agriculture for Future Generations: The Impact of Spectroscopy on an Important Field
February 1st 2024Welcome to our Advancing Agriculture for Future Generations content series! Begin your exploration by checking out a compilation of our articles that spotlight how spectroscopy is revolutionizing the agriculture industry.