Gas Chromatography–Mass Spectrometry (GC–MS) with Cold Electron Ionization (EI): Bridging the Gap Between GC–MS and LC–MS
Gas chromatography–mass spectrometry (GC–MS) with cold electron ionization (EI) is based on interfacing the GC and MS instruments with supersonic molecular beams (SMB) along with electron ionization of vibrationally cold sample compounds in SMB in a fly-through ion source (hence the name cold EI). GC–MS with cold EI improves all the central performance aspects of GC–MS. These aspects include enhanced molecular ions, improved sample identification, an extended range of compounds amenable for analysis, uniform response to all analytes, faster analysis, greater selectivity, and lower detection limits. In GC–MS with cold EI, the GC elution temperatures can be significantly lowered by reducing the column length and increasing the carrier gas flow rate. Furthermore, the injector temperature can be reduced using a high column flow rate, and sample degradation at the cold EI fly-through ion source is eliminated. Thus, a greater range of thermally labile and low volatility compounds can be analyzed. The extension of the range of compounds and applications amenable for analysis is the most important benefit of cold EI that bridges the gap with LC–MS. Several examples of GC–MS with cold EI applications are discussed including cannabinoids analysis, synthetic organic compounds analysis, and lipids in blood analysis for medical diagnostics.
Gas chromatography–mass spectrometry (GC–MS) is a central analytical technique for a broad range of applications and disciplines. A major strength of GC–MS with its standard electron ionization (EI) ion sources is its ability to provide easy sample identification with names and structures at the isomer level through the use of 70 eV EI libraries.
However, GC–MS suffers from two major limitations: First, only a relatively small range of volatile, thermally stable compounds are amenable for analysis, and second, EI mass spectra suffer from a frequent absence or weakness of the molecular ions. Absent or weak molecular ions result in a reduced confidence level in the sample identification via the library and make it impossible to identify the majority of compounds that are not in the library. As a result of these two limitations, the use of liquid chromatography–mass spectrometry (LC–MS) is growing.
Several other softer ion sources were developed for GC–MS, including field ionization (FI), photoionization (PI), chemical ionization (CI), and atmospheric pressure chemical ionization (APCI). Chemical ionization can serve for the provision of protonated molecular ions, and CI ion sources sometimes supplement EI ion sources in GC–MS. However, CI usually requires venting the GC–MS system and the manual replacement of the ion source; therefore, given it is less sensitive than EI, CI is incompatible with library-based sample identification and ineffective with several important classes of compounds with low or negative proton affinity. In addition, its closed ion source design further restricts the volatility range of compounds accessible for analysis and promotes increased peak tailing and sample degradation. CI also adds cost to a GC–MS system. Thus, CI, like the other “soft” ion sources, is rarely used. Another claim for a softer ion source is to use low electron energies, but the use of low eV EI does not provide molecular ions for compounds in which it is absent in 70 eV EI (1).
In the last thirty years, we developed and explored the performance capabilities of a new type of GC–MS based on the use of supersonic molecular beams (SMB) for interfacing the GC and MS instruments and as a medium for electron ionization of vibrationally cold sample compounds in the SMB (cold EI), in a contact-free fly-through ion source (2–15).
A supersonic molecular beam is formed by the expansion of gas through a small pinhole or shaped nozzle into a vacuum chamber. In this adiabatic expansion, the carrier gas (typically helium) and sample molecules obtain the same final velocity so that the sample compounds are accelerated to the carrier gas velocity. The uniform velocity ensures slow intrabeam collisions, resulting in internal vibrational–rotational cooling of the sample compounds. SMBs are characterized by a few features including: a) vibrational and rotational cooling of the seeded sample molecules; b) unidirectional molecular motion in space with heavy species concentration along the beam axis (jet separation); c) controlled amount of sample species kinetic energy up to 20 eV; and d) high carrier gas flow rate compatibility up to 100 mL/min.
GC–MS with cold EI improves all the central performance aspects of GC–MS. This includes improving sample identification via the provision of enhanced molecular ions combined with improved mass spectral isomer and structural information (2–5). It also makes a significantly extended range of compounds (and applications) amenable for analysis (6) and the GC–MS process effectively fast (5,7). There is improved sensitivity, particularly for sample compounds that are difficult to analyze (8,9). Lastly, very good response uniformity is provided for improved quantitation and for the measurement of chemical reaction yields (10).
Cold EI began in 1990 with the discovery of the large enhancement of the molecular ion of cholesterol and bromopentane upon its cooling in SMB (11–13). In 1994, GC–MS with cold EI was born (14) via the use of ultrafast isothermal GC and, shortly after that, the first paper on GC–MS with cold EI analysis of hydrocarbons using a standard GC instrument was published (2). In 2010, Aviv Analytical commercialized GC–MS with cold EI based on the conversion of an Agilent 5975 GC–MS instrument into GC–MS with cold EI (15). Mass spectrometry of vibrationally cold molecules in free jet or SMB was also explored by several other groups (16–26).
Experimental: The GC–MS with Cold EI Systems
Figure 1: A schematic diagram of a GC–MS with cold EI, a supersonic molecular beam interface, and fly-through ion source. It is based on the Aviv Analytical conversion of a previous design (Agilent 7890 GC + 5977 MSD) into a GC–MS with cold EI. The schematic diagram of the SMB interface components are drawn approximately to scale and main component names are included. Further explanations are given in the text.
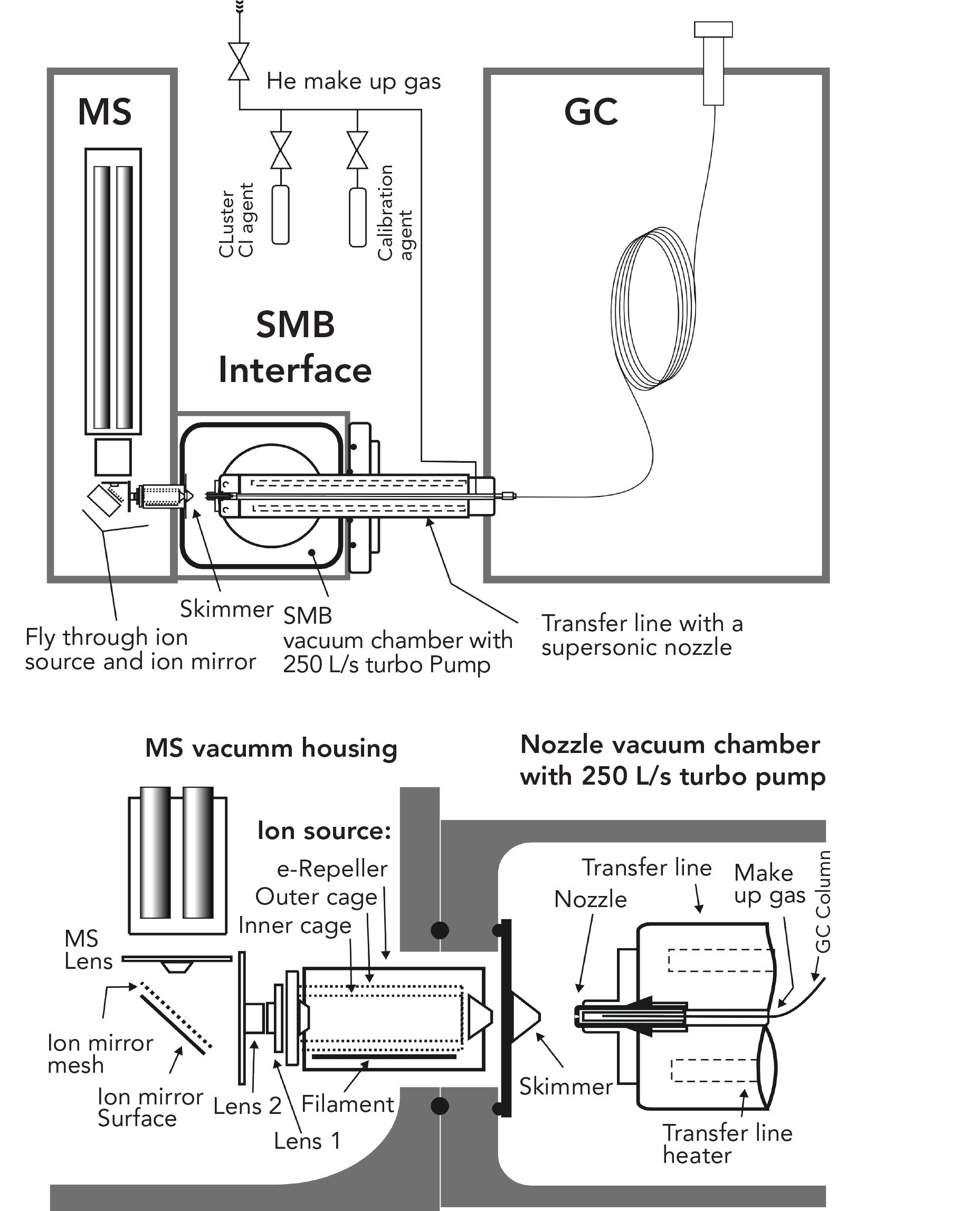
Figure 1 shows a schematic of the Aviv Analytical 5977-SMB GC–MS with cold EI system. The GC column output is mixed with helium make-up gas (typically 60 mL/min total for cold EI or ~5 mL/min total for classical EI with SMB), at typically 830 mBar pressure in front of a supersonic nozzle. The supersonic nozzle itself is based on a shaped pinhole with a 100 μm diameter made from ruby ceramic, located at the end of a heated, temperature-controlled transfer line. The helium make-up flow can be mixed (via the opening of a respective solenoid valve) with perfluorotributylamine (PFTBA) vapor for periodic system tuning and calibration. Sample compounds seeded in helium expand from the supersonic nozzle into a nozzle vacuum chamber that is differentially pumped by an Agilent TwisTorr 304 turbo molecular pump with 250 L/s pumping speed. The expanded sample compounds form a supersonic molecular beam with vibrationally cold molecules. These vibrationally cold sample compounds are skimmed by a 0.8 mm skimmer and are collimated in a second differentially pumped vacuum chamber. A Pfeiffer 250 L/s turbomolecular pump is used as provided by Agilent for its 5977 MS for the pumping of the ion source and MS vacuum chamber. The molecular beam with cold sample compounds fly through a dual cage EI ion source (27) where these beam species are ionized by 70 eV electrons with 5–10 mA emission current. The ions are focused by an ion lens system, deflected 90o by an ion mirror and introduced into the original Agilent 5977 mass analyzer of the GC–MS system. The ion lens system also serves for vacuum background filtration through low positive voltage biasing of one lens element that repels low kinetic energy vacuum background ions while transmitting the energetic SMB ionized species. The 90o ion mirror is separately heated and serves to keep the mass analyzer clean and to suppress mass-independent neutral noise. The ions that exit the Agilent MS are detected by the Agilent triple-axis ion detector and the data is processed by the software.
Main Beneficial Features of Cold EI
Enhanced Molecular Ions for Improved Sample Identification
Cold EI is the name that we gave to electron ionization of vibrationally cold molecules in SMB. With SMB the sample compounds expand from the supersonic nozzle while they are at the nozzle temperature (typically 250–300 oC). Soon after the expansion, the sample compounds are vibrationally and rotationally cooled while reaching the helium (being the majority) velocity (3–5,13). The sample molecules fly through the dual cage ion source without scattering from its walls and, as a result, are ionized while being vibrationally cold.
Figure 2: A comparison of 18 eV cold EI mass spectrum of (a) n-C24H50 with (b) its 70 eV cold EI mass spectrum, and (c) its NIST library mass spectrum. This figure further includes the comparison of (d) 18 eV cold EI mass spectrum of a branched isoprenoid squalane hydrocarbon C30H62 with (e) its 70 eV cold EI mass spectrum, and (f) its NIST library mass spectrum.
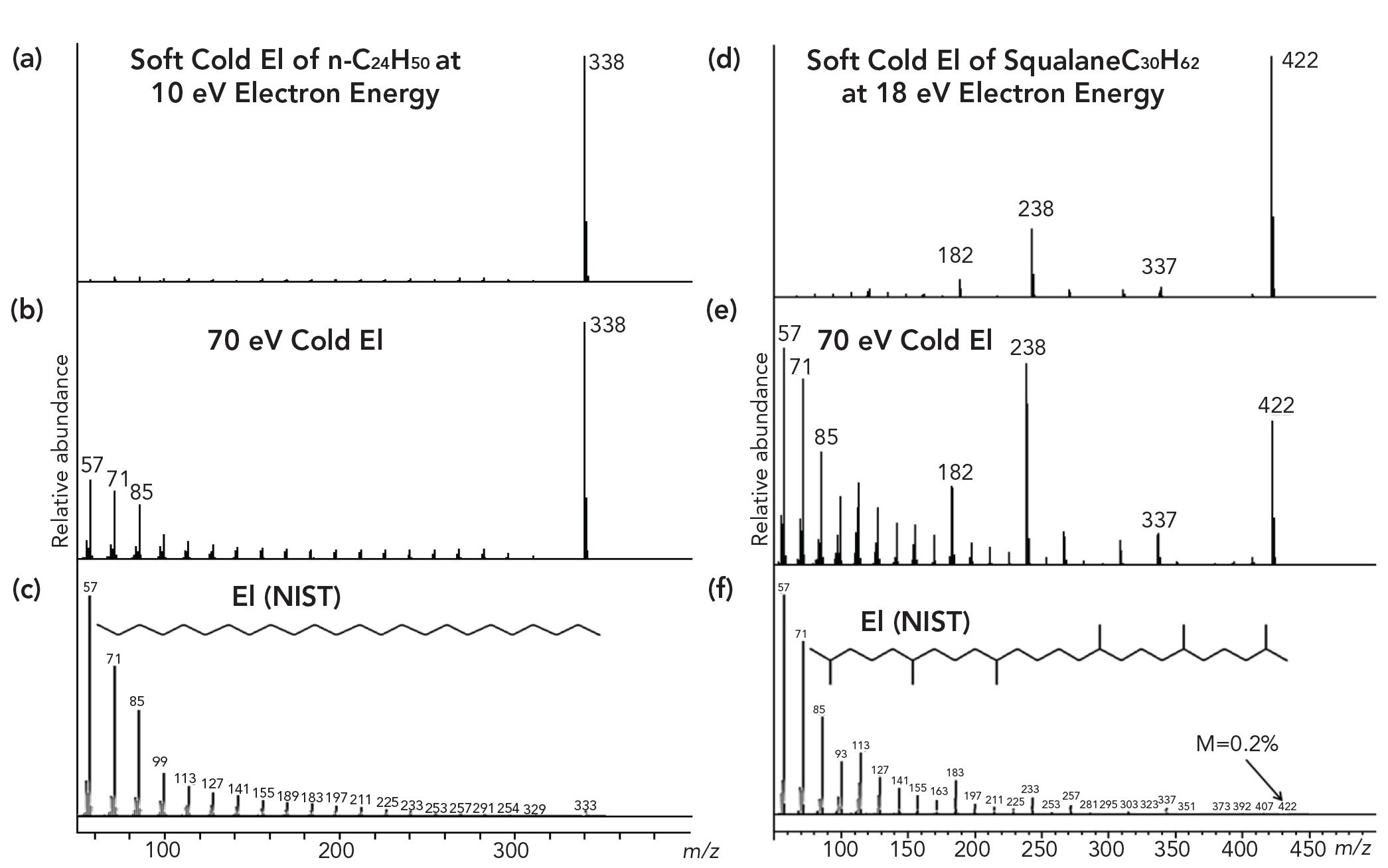
Figure 2 demonstrates the effect of vibrational cooling on EI mass spectra via the comparison of 18 eV cold EI mass spectrum of n-C24H50 (upper left trace) with its 70 eV cold EI mass spectrum (middle left trace) and its NIST library standard EI mass spectrum (lower left trace). Note that the relative abundance of the molecular ion is increased by over a factor of 100 in cold EI. As also shown, at 18 eV the molecular ion is further enhanced via the suppression of fragment ions but such a low eV cold EI mass spectrum suffers from incompatibility with the NIST library identification and the total ion count signal is weaker thus we consider the 70 eV cold EI MS as the most useful MS ionization technique. On the right side of Figure 2, the effect of vibrational cooling on EI mass spectra is demonstrated for squalane which is a highly branched isoprenoid C30H62 hydrocarbon. The 18 eV cold EI mass spectrum of squalane (upper right trace) is compared with its 70 eV cold EI mass spectrum (middle right trace) and its NIST library standard EI mass spectrum (lower right trace). As for n-C24H50, we observe a major SMB cooling effect on the squalane mass spectra. Figure 2 also shows that cold EI significantly amplifies isomeric, structurally important fragment ions and thus cold EI enables much easier conversion of its mass spectrum into the squalane structure than standard EI.
The availability in cold EI of molecular ions for hydrocarbons and their branched isomers uniquely enables isomer distribution analysis, which is a very important application for the optimization of fuels and oils quality (28).
Sample Identification via Cold EI Mass Spectra and the NIST Library
One of the primary goals and benefits of GC–MS is sample compound identification by the available large MS libraries such as the NIST library, particularly with its excellent sample identification algorithm (29–31). Thus, a common question is asked: “How does the enhanced molecular ion abundance affect the results of National Institute of Standards (NIST) library identification?”
Figure 3: Mass spectra of dimethoate measured with (a) GC-MS system with cold-EI, and (b) a standard EI system.
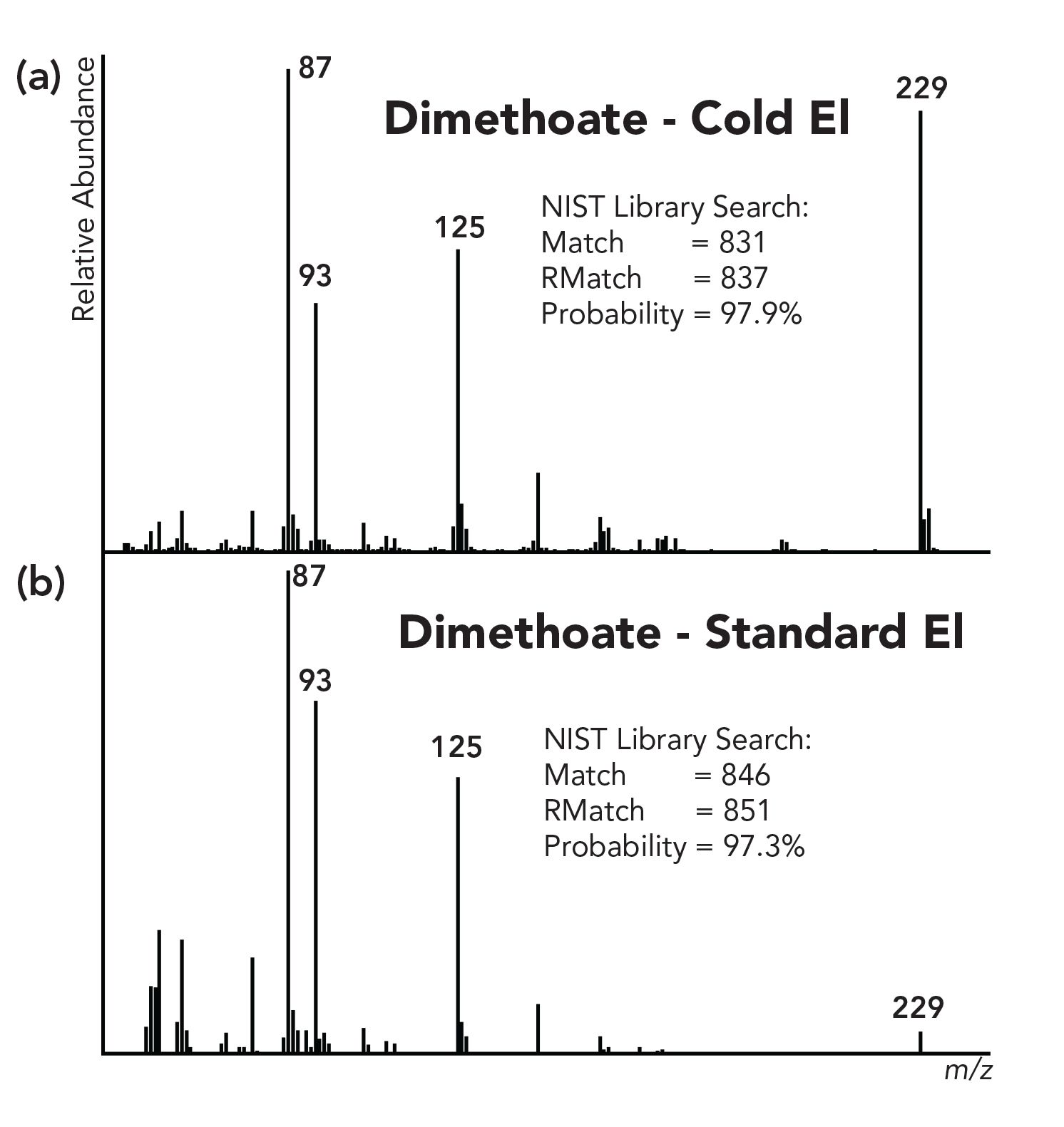
Figure 3 shows the mass spectra of dimethoate, measured with GC–MS with a standard EI system (lower trace) and with GC–MS with a cold EI system (upper trace). The molecular ion’s relative abundance is enriched in cold EI by about a factor of 20. However, the library identification probability is higher for the cold EI mass spectrum (97.9%) than for the standard EI (97.3%) even though cold EI exhibits a lower match factor. In reference 32, we showed a computer simulation of the identification probability versus the molecular ion enhancement factors, and as described, there is a large range of molecular ion enhancement factors that actually improve the NIST library identification. Furthermore, NIST library mass spectra typically show higher abundance molecular ions than in current GC–MS systems. For example, dimethoate has about 8% molecular ion in the NIST library (up to 14% in one replica MS) while our data with an Agilent 5977 with its inert ion source at 250 oC shows only 4.3% relative abundance for standard EI.
We studied 46 compounds and found that cold EI provides on the average higher identification probabilities than standard EI (1).
Accordingly, cold EI is the only soft ionization method that is fully compatible with NIST library–based sample identification.
Classical EI with the SMB System
While the enhancement of the molecular ion in cold EI results in lower matching factors but usually with superior identification probabilities (32,33), for certain compounds and particularly for the identification of certain isomers that exhibit weak isomer mass spectral effects, the availability of “classical EI” mass spectra could be desired. Thus, there is some limited need to supplement and complement cold EI with classical EI in the same GC–MS system ion source.
Figure 4: A demonstration of cold EI and classical EI-SMB mode changing in 10 s. A test mixture was analyzed with 1 ng each hexadecane, methyl stearate, cholesterol, and dotriacontane (n-C32H66) with a 15 m column with 0.32 mm I.D. and 0.1 u DB1HT film. Classical EI with the SMB system was used up to 8.5 min elution time with 1 mL/min column flow rate, 2 mL/min He makeup gas flow rate and 20 oC/min GC oven temperature programming rate followed by cold EI from 8.5 min up to 12 min with 8 mL/min column flow rate, 42 mL/min He make-up flow rate and 40 oC/min GC oven temperature programming rate up to 300 oC. (a) GC-Mass chromatogram in the top of the figure, and (b) classical EI-SMB MS of hexdecane is shown in the bottom left figure and cold EI MS of dotriacontane in the bottom right.

Thus, GC–MS with cold EI provides a “smart EI” ion source with three modes of operation of cold EI, low eV soft cold EI, and classical EI-SMB, while the change from cold EI to classical EI-SMB mode is achieved via a simple method change in a matter of seconds up to a minute via the reduction of the helium make-up cooling gas flow rate. Figure 4 demonstrates classical EI-SMB and cold EI mode changing in 10 s during a single analysis run. A test mixture was analyzed with 1 ng each hexadecane, methyl stearate, cholesterol and dotriacontane (n-C32H66) with mode changing as indicated in the middle of the run at 8.5 min (experimental conditions are in the Figure 4 caption). Accordingly, we conclude that with cold EI there is no need to have an additional standard EI ion source and if there is a desire to have classical EI mass spectra, the same ion source can be used with a method-based fast mode change and provide classical EI mass spectra with high NIST library matching factors.
Thus, with cold EI, there is no need to have any additional standard EI ion source.
Isotope Abundance Analysis (IAA) for the Provision of Elemental Formula from Cold EI Mass Spectra
Clearly, it is highly desirable to be able to obtain elemental formula from low-cost unit resolution quadrupole based GC– MS systems particularly for the many compounds that are not in the EI–MS libraries. We developed the Tal-Aviv Molecule Identifier (TAMI) software (34–36) that works well with standard EI MS and excels with cold EI MS by providing of abundant molecular ions. TAMI automatically converts the molecular ion group of isotopologues and the measured molecular ion mass (which it can improve its mass accuracy) into elemental formulas, and automatically (zero clicks) confirms or rejects the identification results of the NIST library. As a result, cold EI with TAMI provides an ideal sample identification technology for low cost unit resolution quadrupole MS that is superior to any GC–MS with expensive high-resolution MS (35). We found that isotope abundance analysis alone with 0.14 amu mass accuracy of quadrupole MS in centroid mode is equivalent in its ability to provide the correct elemental formula to 5 ppm accurate mass while if it is used in profile mode with a mass tune function, TAMI can provide elemental formula equivalent to high resolution mass spectrometers with 1 ppm mass accuracy that do not consider the isotope abundance (such as orbital trap instruments) (36).
Extending the Range of Compounds Amenable for Analysis: Bridging the Gap Between GC–MS and LC–MS
The recognized and well-known Achilles heel of GC–MS is its limited range of volatile and thermally stable compounds that are amenable for analysis. This limitation emerges from sample degradation induced by the GC injector, column, or ion source, or a lack of sufficient vaporization.
GC–MS, with cold EI, enables a significant extension of the range of compounds amenable for analysis via the use of short columns with high column flow rates in combination with full elimination of intra-ion source degradation (5,6). GC–MS with cold EI provides the ultimate range of compounds amenable for GC–MS analysis and thus effectively bridges the gap between standard GC–MS and LC–MS. The key parameter for this capability is the use of very high column flow rates, which in combination with the use of shorter columns leads to significantly lower elution temperatures (up to 200 °C lower than in standard GC–MS), while the provision of enhanced molecular ions compensates for the traded GC separation. High column f low rates further reduce intra-injector liner degradation (lower GC injector temperatures and reduced liner residence time) and intra-ion source sample dissociation is inherently avoided due to the cold EI fly-through ion source geometry (5,6,27).
The expanded GC–MS range of compounds amenable for analysis can be separated into three groups: an extended range of thermally labile compounds, an extended range of low volatility compounds, and an extended range of polar compounds.
Figure 5: The analysis of reserpine (C33H40N2O9, MW = 608) by GC–MS with cold EI. (a) GC-Mass chromatogram shown in top figure, and (b) cold EI MS shown in bottom figure.
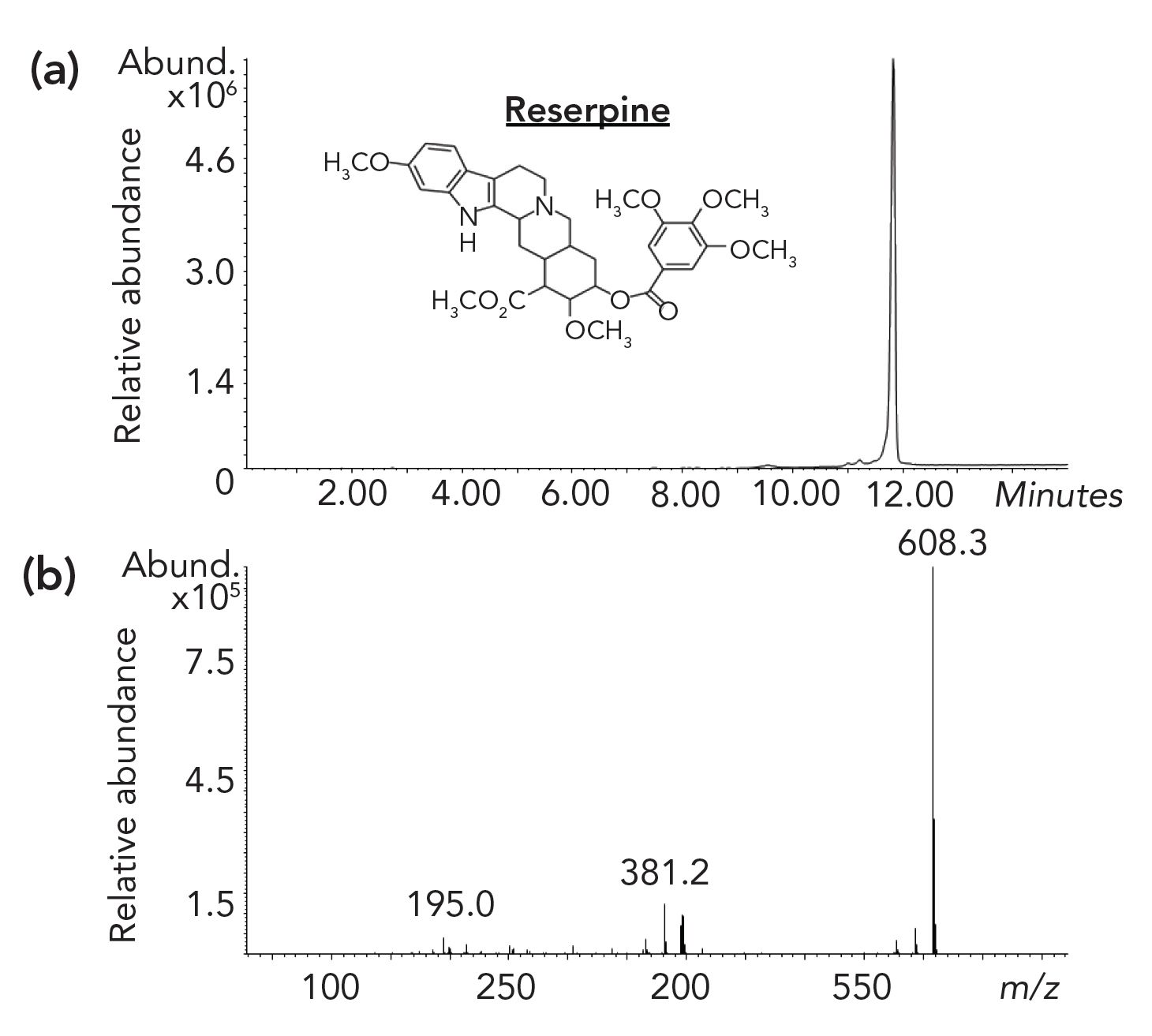
Figure 5 demonstrates the analysis of the polar compound reserpine (C33H40N2O9, MW = 608) by the 5975-SMB GC–MS with cold EI. Although reserpine is the LC–MS industry standard specification compound, GC–MS with cold EI is the only GC–MS that can analyze this large polar compound and it provides a dominant molecular ion. Thus, reserpine serves as an example of the extended range of polar compounds amenable for GC–MS analysis by cold EI.
Figure 6: An illustration that demonstrates our perception of the average total ion count (TIC) and molecular ion signal dependence on the sample compound mass.

Figure 6 shows an illustration that demonstrates our perception of the average total ion count (TIC) and molecular ion signal dependence on the sample compound mass obtained with cold EI and with standard EI. From this figure, one can calculate the average sensitivity gain of cold EI with the 5977-SMB GC–MS with cold EI versus sample mass, which at some point is transformed into extended range of compounds amenable for analysis.
Sensitivity and Limits of Detection
Detectability is considered to be one of the most important GC–MS parameters used to characterize the quality and value of an instrument. However, detectability is often misrepresented because all the major vendors use octafluoronaphthalene (OFN) for their specifications in selected-ion monitoring (SIM) or full scan reconstructed SIM (RSIM) modes and instrument detection limit (IDL). OFN specifications are misleading for real samples since OFN is one of the easiest compounds to detect. GC–MS with cold EI can obtain for one pg OFN S/N > 10+6 because it has practically no noise (37). However, GC–MS detectability should be evaluated by its performance using a mixture of several selected “hard to analyze” compounds, along with OFN (8).
Figure 7: A comparison of (a) 10 pg on column cholesterol, and (b) n-C32H66 signals in SIM mode on the corresponding molecular ions for sensitivity evaluation of GC–MS with (a,b) standard EI (Inert ion source) and (c,d) cold EI. As demonstrated, cold EI provides for cholesterol (m/z = 386.4) over 600 times better S/N while for n-C32H66 on its molecular ion the Cold EI S/N is over 2000 times better.
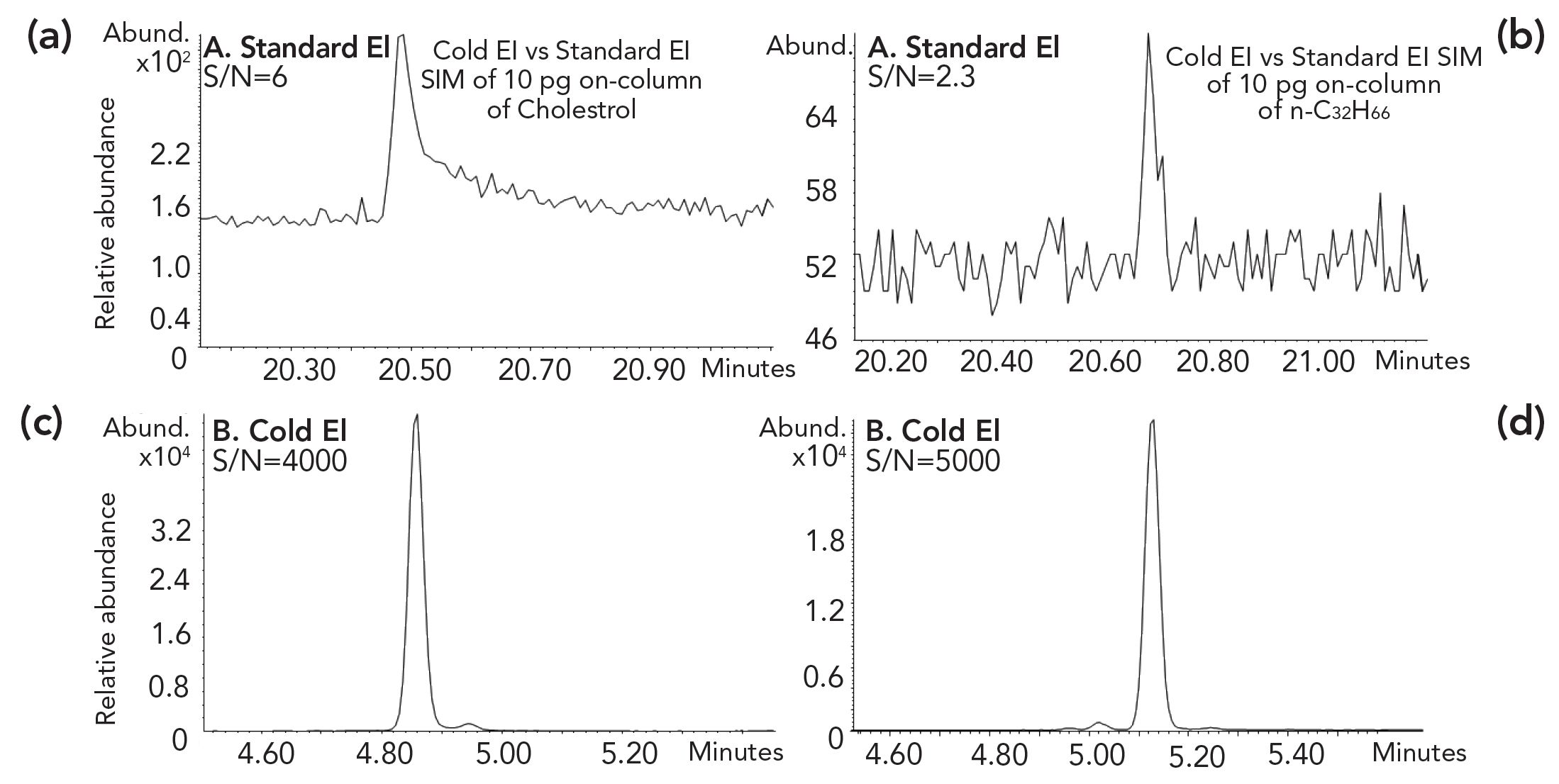
Thus, Figure 7 shows that cold EI exhibits much better S/N than standard EI for compounds such as cholesterol and n-C32H66. As demonstrated, cold EI provides for 10 pg cholesterol in SIM on its molecular ion (m/z = 386.4) over 600 times better S/N while for n-C32H66 the cold EI S/N is over 2000 times better. The 600 times superior cold EI S/N for cholesterol emerges from three main reasons: a) The molecular ion is enhanced in its absolute abundance by about 30 times; b) the total ion chromatogram (TIC) signal of cholesterol is reduced due to ion source peak tailing over 10 times compared with those of early eluters such as OFN; and c) the noise on the column bleed plateau where cholesterol elutes in standard EI is increased compared with that on OFN molecular ion while it is very low at the cholesterol elution time in cold EI. With n-C32H66 the S/N gain of cold EI is higher also due to greater enhancement of the molecular ion. We note that the obtained S/N for cholesterol is further reduced with the Agilent high-efficiency source (HES) despite its high ionization yield due to its lower molecular ion abundance and greater ion source peak tailing. For these same reasons, n-C32H66 was not detected by the HES ion source in SIM at 10 pg on column amount.
Selected Applications of GC–MS with Cold EI
Since the development of GC–MS with cold EI hundreds of applications have been explored with it as listed in part in reference 38. However, here we briefly describe three applications that we consider interesting and demonstrating “bridging the gap between GC–MS with standard EI and LC–MS.”
Cannabis and Cannabinoids Analysis
The analysis of cannabis-based drugs is currently a highly popular subject. GC–MS with standard EI serves mostly for terpenes in cannabis analysis as well as for pesticides analysis while LC–UV or LC–MS serve for target cannabinoids analysis. However, universal untargeted cannabinoids analysis is not performed due to unavailability of appropriate tools for such analysis given that compounds with OH groups tend to tail and decompose on the metallic surface of standard EI ion sources. Furthermore, diglycerides and triglycerides are currently ignored. The most widely known and potent compounds in cannabis are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) that were initially explored and the benefits of which promoted in Israel by Mechoulam and his group (39). Cannabis-related compounds are described by Brenneisen (40) who lists and describes 58 cannabinoids. Accordingly, an important yet unmet challenge is analyzing untargeted cannabinoid compounds in various brands of cannabis and correlating the cannabis brands’ medical effectiveness with their cannabinoid compound content (this is known as the entourage effect).
Figure 8: Cannabis EP-1, a drug for children with autism spectrum disorder. GC–MS with cold EI mass chromatogram (a) Zoomed around the elution time of cannabinoids, and (b) cold EI mass spectrum of cannabidivarin. Abbreviated names and molecular weights of selected cannabinoids and other detected compounds are indicated. (a) GC top figure, and (b) MS shown in bottom figure.
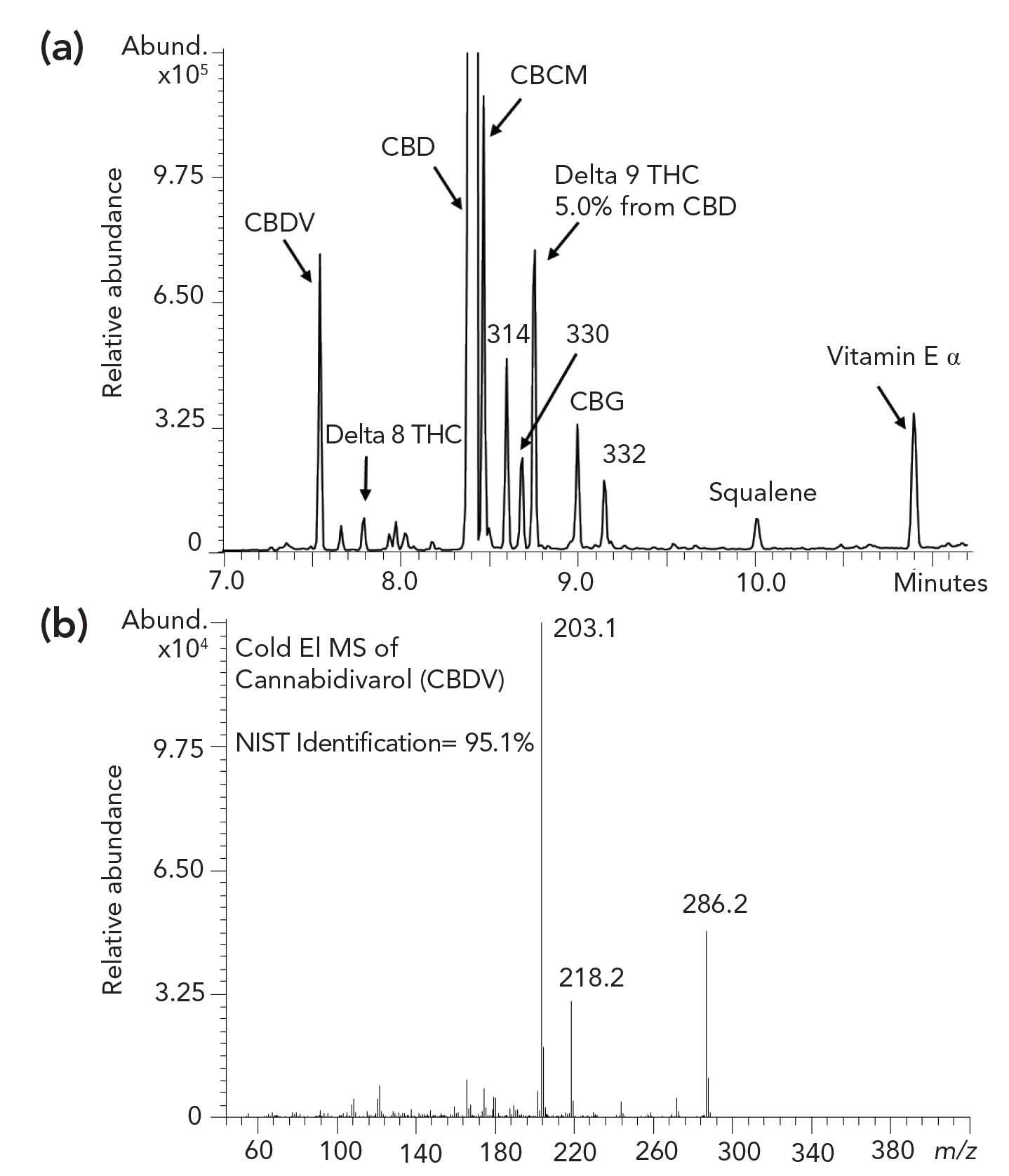
Figure 8 shows the analysis of Cannabis EP-1, which is a drug for children with autism spectrum disorder. A GC–MS with cold EI mass chromatogram zoomed around the elution time of cannabinoids is shown in the upper trace while cold EI mass spectrum of cannabidivarol is shown at the bottom trace. As shown, cold EI analyzes the full cannabinoid content and provides indications to what is unique in it that makes it an effective drug for children with autism spectrum disorder. We measured that the THC/CBD ratio is 5.0%, in agreement with analysis by LC with diode array and calibration measurement (note that GC–MS with cold EI uniquely provided this ratio measurement without calibration) and found that the cannabidivarol (CBDV) abundance is relatively high compared with other cannabis extracts. In addition, we also found vitamin E in the form of alpha tocopherol, as indicated in Figure 8. The road is now open to use cold EI to explore the entourage effect to optimize the efficacy of cannabis-based drugs.
Organic Chemistry Service Analysis
One of the most widely used GC–MS applications is service analysis in universities and research institutes. There are about 28,000 universities worldwide and most have chemistry or life science departments with at least one (or few) service GC–MS systems. In universities, an important goal is the measurement and optimization of organic chemical reactions. Although GC–MS is being used in universities, its performance is weak and LC–MS is preferred. GC–MS with cold EI uniquely provides several very important benefits for service GC–MS (10): First, it provides an extended range of compounds amenable for analysis, including much larger and more labile synthetic compounds. Second, trustworthy molecular ions are present for large organic compounds, which are then converted into elemental formulae with our TAMI software (34–36). Third, GC–MS with cold EI also allows for much faster analysis (8 min) for semi online reaction monitoring feedback. Fourth, uniform compound-independent responses are provided as needed for the provision of chemical reaction yields (10). And lastly, compound analysis that cannot be ionized in LC–MS by its various ion sources can be ionized here.
Figure 9: GC–MS with cold EI analysis of an organometallic compound with a weak coordinative bond (a,b). GC shown in upper two figures, and (c) MS shown in bottom figure. Samples and application information was kindly given by Dr. Qing Ma and Professor Michael Gozin’s group at Tel Aviv University.
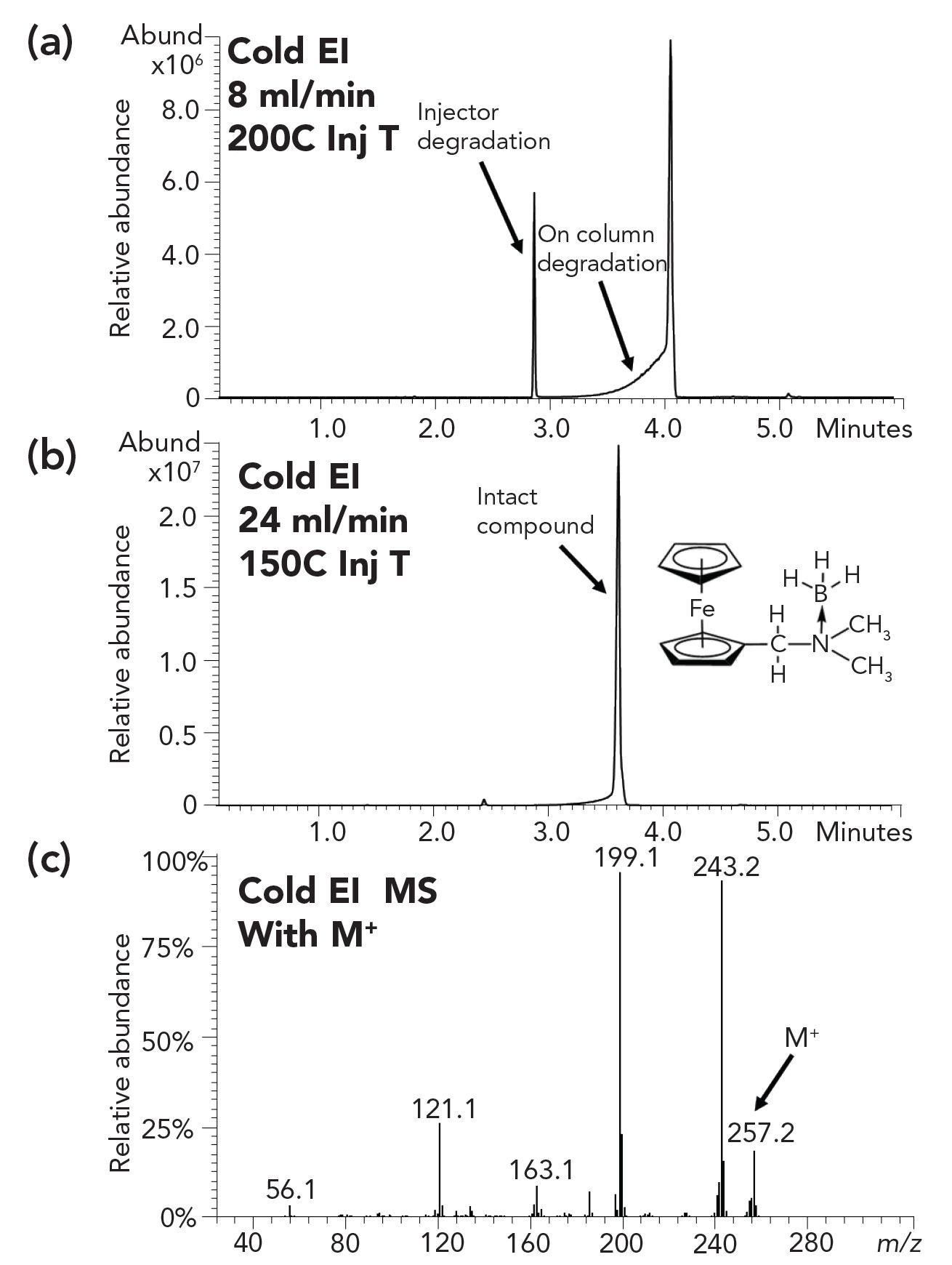
Figure 9 shows an example of GC–MS with cold EI analysis of an organometallic compound with a weak coordinative bond. This analysis is impossible with GC–MS with standard EI and failed with LC–MS. We used an 8 mL/min column flow rate and a 200 oC injector temperature to obtain the upper mass chromatogram in which the sample compound partially degraded at the injector and at the column (hump prior to elution). Upon a column flow rate increase to 24 mL/min and an injector temperature reduction to 150 oC as shown in the middle mass chromatogram, a practically clean peak was obtained with cold EI MS showing the correct molecular ion (bottom mass spectrum).
This example demonstrates the type of compounds that can be analyzed only by GC–MS with cold EI and no other MS instrumentation and thus as bridging the gap between GC–MS with standard EI and LC–MS (10).
Lipids in Human Blood Analysis for Medical Diagnostics
The analysis of cholesterol and triglycerides in human blood is one of the most important and widely used medical diagnostics tests. These tests are vital for the control and monitoring of various heart diseases and other medical issues. However, currently these tests provide limited information on protein-bound cholesterol (LDL and HDL) and not on the blood majority free cholesterol and various cholesteryl esters plus on the total glycerides and not on their type and their fatty acid chemical compositions.
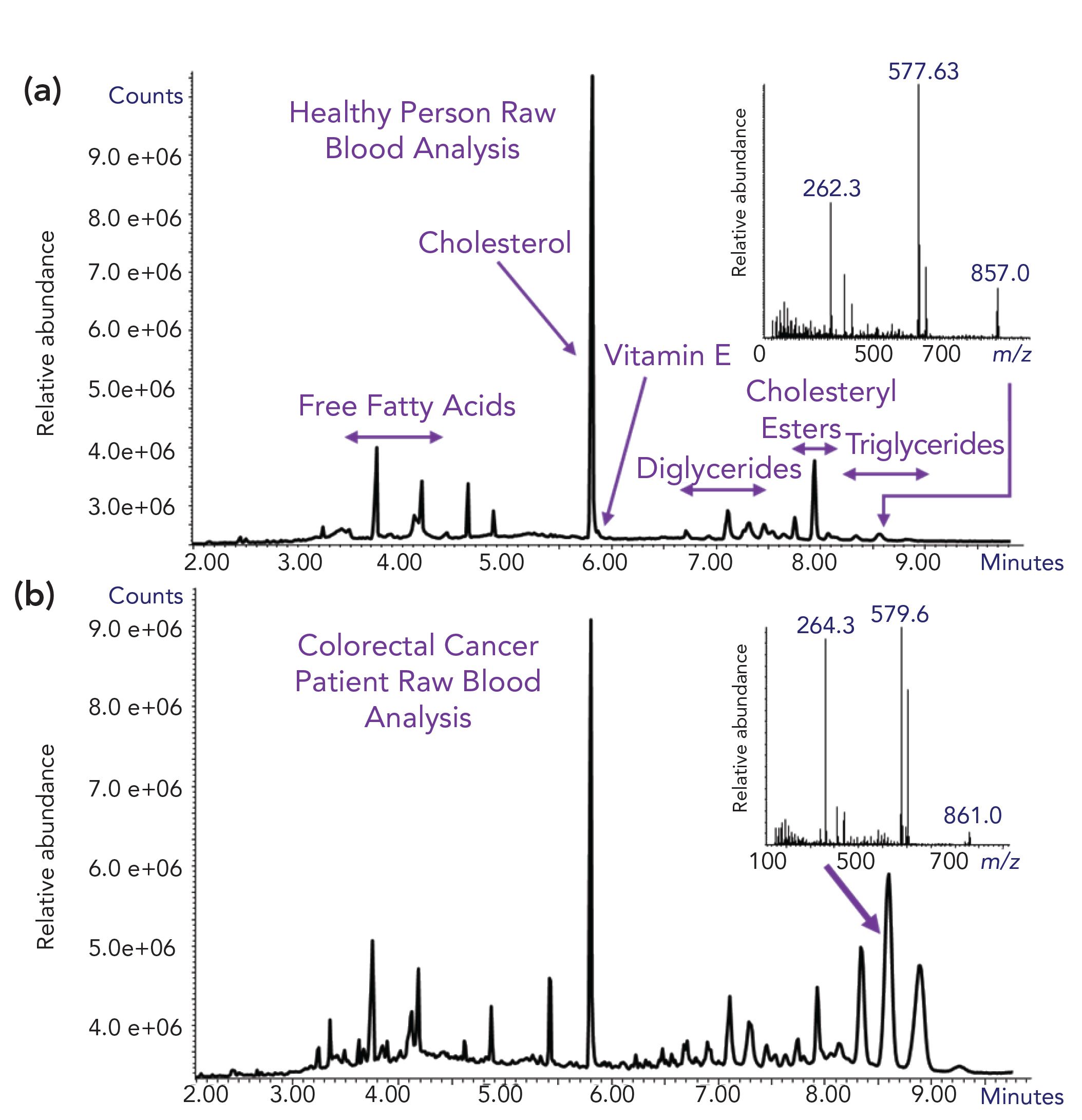
We are currently engaged in the development of a new type of “Blood-Probe” device for the sampling of raw blood and its insertion for analysis with GC–MS with cold EI (41). BloodProbe is a ChromatoProbe (42) vial holder that was modified to hold a thin glass rod (the same in our Open Probe Fast GC– MS (43,44) (the Agilent name “Quick-Probe” [45]) for its insertion into a ChromatoProbe device followed by GC–MS with cold EI analysis. As shown in Figure 10, we can obtain information on the full range of nonpolar lipids and with no sample preparation and a simple diabetes lancet being used to (minimally invasive) draw 2.0 μL blood that was taken with a micro pipette and deposited on the BloodProbe thin glass rod for its introduction as a dried raw blood spot. Another benefit is that only 2.0 μL blood was used, thus avoiding the use of a syringe in hand veins that collects several mL (10– 30) of blood. Lastly, the instrument and method used provide far greater information about lipids in blood information at the molecular level in comparison with currently used methods.
Figure 10: GC–MS with cold EI total ion mass chromatograms and cold EI mass spectra of two human raw blood samples obtained with a diabetes lancet, deposited on a BloodProbe device and inserted into a ChromatoProbe mounted on a GC–MS with cold EI. The names of the various compounds and compound families are indicated and all with molecular ions. Cold EI mass spectra of selected triglycerides are shown at the inserts. Note the differences between the two human samples. GC chromatograms are shown as large figures with mass spectra as inserts.
Discussion and Conclusions
GC–MS with cold EI has over 60 benefits and advantages over GC–MS with standard EI (46,47) without any downside and thus it is leading the way to the future of GC–MS. GC–MS with cold EI is bridging the gap with LC–MS and serves for the analysis of an extended range of compounds and applications that are either difficult by standard GC–MS or unamenable for analysis by GC–MS with standard EI or even LC–MS. The advantages of GC–MS with cold EI are based mostly on the ionization of vibrationally cold molecules during their axial flight path in a fly-through ion source and due to the ability to analyze samples with high column flow rates up to 100 mL/min without affecting sensitivity. We have explored several hundred applications with GC–MS with cold EI. Many of them are described in our publications while in our Advanced GC–MS Blog Journal (38) we describe 57 application notes.
GC–MS with cold EI is characterized by the following main 20 improvements and benefits:
1. Significantly extended range of com- pounds amenable for analysis (6).
2. Enhanced molecular ions and structurally-informative fragment ions (2–6).
3. The only soft ionization method that is fully compatible with NIST library identification (32).
4. Uniform response for improved quantitation and measurement of chemical reaction yields (10).
5. The same ion source provides cold EI, classical EI and low eV soft cold EI mass spectra with method-based mode changing (33).
6. Best provision of elemental formula with the TAMI software by providing enhanced molecular ions for greater range of compounds (34–36).
7. Much faster analysis is enabled (7).
8. Column flow programming is provided without affecting sensitivity (7).
9. Improved sensitivity particularly for compounds that are difficult to analyze (8,9).
10. Lowest MS baseline noise by vacuum background filtration (elimination) and highest (by far) ratio of TIC peaks to column bleed (5).
11. Inherently inert ion source even for low pg range polar compounds (48).
12. No ion source related peak tailing is observed in cold EI for better signal as well as improved chromatography and separation (5).
13. Isomer distribution analysis is uniquely enabled (28).
14. Doubly charged molecular ions are provided for doubled mass spectral range (49).
15. Fastest (by far) ion source response time (5).
16. Improved selectivity and thus reduced matrix interference on the molecular ions.
17. Better linearity and reproducibility at low levels (48).
18. Best compatibility with pulsed flow modulation GCxGC–MS and a range of additional GC–MS enhancement technologies (50,51).
19. GC–MS with cold EI and LC–MS with cold EI can be combined in one MS system with method-based mode changing (52).
20. Robust ion source that rarely needs cleaning.
Naturally, some improvements are more important than others, and we consider the extension of the range of compounds amenable for analysis as the most important advantage of GC–MS with cold EI because it bridges the gap between GC–MS and LC–MS and opens up new areas of analysis and applications.
The combination of so many improvements creates a new and qualitatively superior technology that improves every type of analysis. While GC–MS with cold EI enables new types of analyses and significantly improves challenging analyses, it does not impede on any simple method of analysis (compared with standard EI) and its added cost could be negligible or none in a fully integrated GC–MS with cold EI. Consequently, we believe that GC–MS with cold EI is destined to lead the way for the future of GC–MS.
Acknowledgments
This research was supported by the Israel Science Foundation (grant No 356/15).
References
(1) K.J. Margolin Eren, O. Elkabets and A. Amirav, “A Comparison of Electron Ionization Mass Spectra Obtained at 70 eV, Low Electron Energies and with Cold EI and Their NIST Library Identification Probabilities” J. Mass Spectrom. DOI: 10.1002/jms.4646 (In Press).
(2) S. Dagan and A. Amirav, “Electron Impact Mass Spectrometry of Alkanes in Supersonic Molecular Beams” J. Am. Soc. Mass. Spectrom. 6, 120–131 (1995).
(3) A. Amirav, A. Gordin and N. Tzanani, Rapid. Com. Mass Spectrom. 15, 811– 820 (2001).
(4) A. B. Fialkov, U. Steiner, L. Jones and A. Amirav, Int. J. Mass. Spectrom. 251, 47–58 (2006).
(5) A. Amirav, A. Gordin, M. Poliak, and A. B. Fialkov, J. Mass Spectrom. 43, 141–163 (2008).
(6) A.B. Fialkov, A. Gordin and A. Amirav, J. Chromatog. A 991, 217–240 (2003).
(7) A. Amirav, Chromatographia 80, 295–300 (2017).
(8) A.B. Fialkov, U. Steiner, S.J. Lehotay and A. Amirav, Int. J. Mass. Spectrom. 260, 31–48 (2007).
(9) A. Amirav, “Achieving the Lowest Limits of Identification – GC-MS with Cold EI versus Standard EI with High Efficiency Source” (accessed December 17, 2018). http://blog.avivanalytical.com/2018/12/ achieving-lowest-limits-of.html
(10) A. Amirav, A. Gordin, Y. Hagooly, S. Rozen, B. Belgorodsky, B. Seemann, H. Marom, M. Gozin and A. B. Fialkov, Tetrahedron. 68, 5793–5799 (2012).
(11) A. Amirav and A. Danon, Int. J. Mass Spectrom and Ion Proc. 97, 107–113 (1990).
(12) A. Amirav, J. Phys. Chem. 94, 5200–5202 (1990).
(13) A. Amirav, Org. Mass. Spectrom. 26, 1–17 (1991).
(14) S. Dagan and A. Amirav, Int. J. Mass. Spectrom & Ion Proc. 133, 187–210 (1994).
(15) Aviv Analytical. GC-MS with Cold EI - The Ultimate Performance GC-MS. http:// www.avivanalytical.com/Superson- ic-GC-MS.aspx
(16) H. Kishi, A. Tahara and T. Fujii, Int. J. Mass Spectrom. 209, 133–140 (2001).
(17) K. Hafner, R. Zimmermann, E.R. Rohwer, R. Dorfner and A. Kettrup, Anal. Chem. 73, 4171–4180 (2001).
(18) A. Voloshenko, R. Shelkov, O. Lev and J. Gun, Anal. Chim. Acta. 685, 162–169 (2011).
(19) A. Voloshenko, K.J. Tan, C. Kloc, O. Lev, M. Shelkov, S. Sladkevich, P.V. Prikhodchenko, and J. Gun, Org. Electronics 14, 378–388 (2013).
(20) E. Smolianitski-Fabian, E. Cohen, M. Dronova, A. Voloshenko-Rossin, and O. Lev. Drug Test. Anal. 10, 474–487 (2018).
(21) C. De Jonge, E.C. Hopmans, A. Stadnitskaia, W. Irene, C. Rijpstra, R. Hofland, E. Tegelaar and J. S. Sinninghe Damst, Org. Geochem. 54, 78–82 (2013).
(22) D. Rush, A. Jaeschke, J.A.J. Geenevasen, E. Tegelaar, J. Pureveen, M.D. Lewan, S. Schouten, J. S. Sinninghe Damsté, Org. Geochem. 76, 136–145 (2014).
(23) Y.F. Wong, T.M. Uekane, C.M. Rezende, H.R. Bizzo and P.J. Marriott, J. Chro- matogr. A 1477, 91–99 (2016).
(24) M.P. Levitas, E. Andrews, I. Lurie, and I. Marginean, Forensic Sci. Int. 288, 107– 114 (2018).
(25) J.S. Sinninghe Damsté, W. Irene, C. Rijpstra, E. C. Hopmans, M. J. den Uijl. J.W.H. Weijers and S. Schouten, Org. Geochem. 124, 22–28 (2018).
(26) S. Buchalter, I. Marginean, J. Yohannan, and I.S. Lurie, J. Chromatogr. A 1596, 183–193 (2019).
(27) A. Amirav, A. B. Fialkov, and A. Gordin, Rev. Sci. Instrum. 73, 2872–2876 (2002).
(28) A.B. Fialkov, A. Gordin and A. Amirav, J. Chromatogr. A 1195, 127–135 (2008).
(29) S.E. Stein, J. Am. Soc. Mass Spectrom. 5, 316–323 (1994).
(30) S.E. Stein and D.R. Scott, J. Am. Soc. Mass Spectrom. 5, 859–866 (1994).
(31) S.E. Stein, J. Am. Soc. Mass Spectrom. 10, 770–781 (1999).
(32) T. Alon and A. Amirav, Rapid. Commun. Mass. Spectrom. 29, 2287–2292 (2015).
(33) A. Gordin, A. Amirav and A.B. Fialkov, Rapid. Commun. Mass Spectrom. 22, 2660–2666 (2008).
(34) T. Alon and A. Amirav, Rapid Commun. Mass Spectrom. 20, 2579–2588 (2006).
(35) Aviv Analytical. Tal-Aviv Molecule Identifier (TAMI). http://www.avivanalytical.com/Isotope-Abundance.aspx
(36) T. Alon and A. Amirav, “A Comparison of Isotope Abundance Analysis and Ac- curate Mass Analysis in their Ability to Provide Elemental Formula” Submitted to J. Mass Spectrom.
(37) A. Amirav “OFN Sensitivity Specifications – Are they of any Value or Just a Game” (accessed August 15, 2012). http://blog. avivanalytical.com/2012/08/ofn-sensitivity-specifications-are-they.html
(38) A. Amirav, A.B. Fialkov, and T. Alon. The Multiple Benefits of Cold EI – Leading the Way to the Future of GC-MS. http:// blog.avivanalytical.com/
(39) R. Mechoulam, Science 168, 1159 (1970).
(40) R. Brenneisen, “Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents” From, Forensic Science and Medicine, Marijuana and the Cannabinoids, Edited by: M. A. ElSohly (Humana Press Inc., Totowa, New Jersey, 2007), pp. 17–49.
(41) B. Neumark, G. Shefer and A. Amirav, “Lipids in Raw Blood Analysis for Medi- cal Diagnostics by GC-MS with Cold EI” In Preparation.
(42) A. Amirav and S. Dagan, Europ. Mass. Spectrom. 3, 105–111 (1997).
(43) A. Amirav, U. Keshet, T. Alon and A.B. Fialkov, Int. J. Mass Spectrom. 371, 47–53 (2014).
(44) U. Keshet, T. Alon, A.B. Fialkov and A. Amirav, J. Mass Spectrom. 52, 417–426 (2017).
(45) Agilent. Chemical Analysis, Life Sciences, and Diagnostics. https://www. agilent.com/en/product/gas-chromatography-mass-spectrometry-gc-ms/gc-ms-ion-sources-inlets/quickprobe-gc-ms-system
(46) A. Amirav, A.B. Fialkov and T. Alon, Int. J. Anal. Mass. Spectrom. Chrom. 1, 31–47 (2013).
(47) A. Amirav, A.B. Fialkov and T. Alon, “The Multiple Benefits of Cold EI – Leading the Way to the Future of GC-MS” (accessed May 2 2019). http://blog. avivanalytical.com/2019/05/the-multiple-benefits-of-cold-ei.html
(48) A. Amirav, U. Keshet and B. Belgorodsky, “Linearity Sensitivity and Response Uniformity Comparison of the Aviv Analytical 5975-SMB GC-MS with Cold EI and the Agilent 5977A GC-MS with Standard EI” (accessed May 8 2014). http://blog.avivanalyti- cal.com/2014/05/linearity-sensitivity-and-response.html
(49) K. Margolin-Eren, A.B. Fialkov, U. Keshet, S. Tsizin and A. Amirav, J. Am. Soc. Mass Spectrom. 31, 347–354 (2020).
(50) M. Poliak, M. Kochman and A. Amirav, J. Chromatogr. A. 1186, 189–195 (2008).
(51) M. Poliak, A.B. Fialkov and A. Amirav, J. Chromatogr. A 1210, 108–114 (2008).
(52) S. Tsizin, A.B. Fialkov and A. Amirav, “Electron Ionization Mass Spectrometry for Both Liquid and Gas Chromatography in One System without the Need for Hardware Adjustments” J. Am. Soc. Mass Spectrom. 55, e4516 (2020). DOI: 10.1021/jasms.0c00136.
Aviv Amirav is with the School of Chemistry at Tel Aviv University in Tel Aviv, Israel and also with Aviv Analytical Ltd, in Hod Hasharon, Israel. Alexander B. Fialkov, Ksenia J. Margolin Eren, Benny Neumark, Oneg Elkabets, Svetlana Tsizin, Alexander Gordin, and Tal Alon are all with the School of Chemistry at Tel Aviv University in Tel Aviv, Israel. Direct correspondence to: amirav@tau.ac.il
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.