High-Throughput Analysis of Volatile Compounds in Air, Water, and Soil Using SIFT-MS
Special Issues
For the BTEX compounds, detection limits in the single-digit parts-per-billion concentration range (by volume) are readily achievable within seconds using selected ion flow tube mass spectrometry (SIFT-MS), because sample analysis is achieved without chromatography, preconcentration, or drying.
Since its introduction in 1995, selected ion flow tube mass spectrometry (SIFT-MS) has found many applications in research and industry, together with substantial peer-reviewed literature. In this article, we describe a new application of SIFT-MS that is suited to the contract laboratory, where the direct analysis provided by SIFT-MS may have significant benefits because of its accelerated sample throughput. Using the environmentally significant BTEX compounds (benzene, toluene, ethylbenzene, and the xylenes) as a case study, we demonstrate high-throughput analysis from several matrices (air, water, and soil). For the BTEX compounds, detection limits in the single-digit parts-per-billion concentration range (by volume) are readily achievable within seconds using SIFT-MS, because sample analysis is achieved without chromatography, preconcentration, or drying. For the first time with SIFT-MS, we also present a calibration approach that enables speciation of ethylbenzene from the xylenes in real time.
A number of different technologies have been employed to monitor the aromatic hydrocarbons benzene, toluene, ethylbenzene, and xylenes (collectively known as BTEX) for different applications. Benzene, toluene, and ethylbenzene have been linked with cancers (1–6), with a common source of exposure being land contaminated by oil spillage (7,8). The importance of analyzing these species in environmental matrices, including air, water, and soil, is high and various reference methods have been developed (9–12).
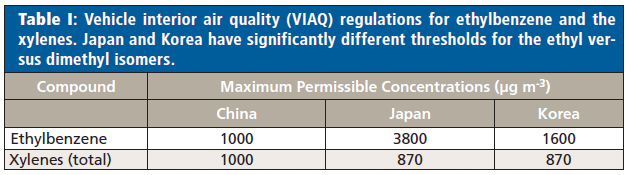
Chromatographic techniques have been the traditional choice-in particular, gas chromatography (GC) with various detectors (most notably flame ionization detection [FID], photoionization detection [PID], and mass spectrometry [MS]). However, GC is usually slow, because of the time-consuming chromatographic separation of analytes, so direct analysis techniques are frequently being evaluated. Among these, direct mass spectrometry (DMS) techniques have come to prominence because of their ability to analyze samples much more rapidly than GC, and with better selectivity than spectroscopic techniques. However, DMS techniques have struggled to distinguish ethylbenzene from the xylene isomers, so their measurement has been reported as a total concentration of "ethylbenzene plus xylenes." Where speciation is required, methods using chromatographic separation must be retained, which means off-line analysis. For example, in the European Union-unlike the United States-chromatography would have been essential because regulators have imposed different occupational exposure limits (OELs) for ethylbenzene and the xylenes (time-weighted averages [TWAs] of 100 and 50 ppm, respectively) (13). Likewise, recent vehicle interior air quality (VIAQ) regulations from Japan and Korea impose quite different permissible emission limits on these compounds-albeit the inverse of the EU workplace regulations (Table I).
With the increasing need to rapidly speciate these isomers-whether in the laboratory or in-process monitoring-enhanced performance of DMS is required, rather than chromatography. Clearly, higher-resolution MS is not a solution, because the elemental composition is the same (C8H10). The need is to utilize an ionization approach that yields different product ions. With a portfolio of eight standard rapidly switchable reagent ions, selected ion flow tube mass spectrometry (SIFT-MS) is the ideal technique with which to seek a solution to distinguishing ethylbenzene from xylenes in real time.
In this article, we describe a new approach to resolving ethylbenzene from the xylene isomers, by utilizing their different reactivities with the O2+ reagent ion of SIFT-MS. Based on calibration, this methodology enables the SIFT-MS technique to achieve direct, real-time speciation of the xylenes from ethylbenzene. We applied this comprehensive approach to BTEX analysis to direct measurement of ambient air, water headspace, and methanolic extraction from soil. We find that this method is successful for air and water, whereas for methanolic extraction only a total can be reported due to methanol residues consuming the O2+ reagent ion.
Methods
SIFT-MS
SIFT-MS (14,15) is a real-time analytical technique for direct, comprehensive gas analysis to ultratrace levels (16). Data obtained by SIFT-MS instruments compare well with those obtained using the accepted chromatographic method for volatile organic compound (VOC) analysis (17).
SIFT-MS uses soft, precisely controlled chemical ionization coupled with MS detection to rapidly quantify VOCs to low parts-per-trillion concentrations by volume (pptv). Eight chemical ionization agents (reagent ions) are now available in commercial SIFT-MS instruments: H3O+, NO+, O2+, O-, O2-, OH-, NO2-, and NO3- (18). These reagent ions react with VOCs and inorganic gases in well-controlled ion–molecule reactions, but they do not react with the major components of air (N2, O2, and Ar). This ionization approach enables SIFT-MS to analyze air at trace and ultratrace levels without preconcentration.
Rapid switching between the eight reagent ions provides very high selectivity. The key benefit of the additional ions is not primarily in the number of reagent ions, but in the multiple reaction mechanisms that provide additional independent measurements of each compound, delivering unparalleled selectivity and detection of an extremely broad range of compounds in real time.
In this study, a Voice200ultra SIFT-MS instrument (Syft Technologies) was used.
Automated Headspace Analysis
Autosampler integration enables the direct gas analysis provided by SIFT-MS to be applied for rapid analysis of discrete samples. Autosamplers have been integrated most commonly with chromatographic analytical techniques, where rapid injection is required to achieve good chromatographic separation. However, SIFT-MS analyzes samples continuously (since chromatography is eliminated), so it requires steady sample injection of the gas sample for the duration of the analysis.
The data presented here were obtained using an integrated Gerstel Multipurpose Sampler (MPS) autosampler, which has proved the best-suited commercial autosampler system for integration with the SIFT-MS technique. Headspace analysis was carried out from 20-mL sample vials on a standard Gerstel vial rack. Gerstel's Maestro control software has the ability to overlap parts of the preparation and injection sequence, leading to highly efficient sample scheduling and increased sample throughput.
Headspace Analysis of Water
BTEX and chloroform primary standard solutions were prepared at 1000 ppm in methanol. Working standards were then prepared, via serial dilution, in 10 mL of deionized water in 20-mL headspace vials. Standard solutions were incubated at 60 °C for 15 min. The headspace was sampled using a 2.5-mL headspace syringe, and then injected into the SIFT-MS instrument at a flow rate of 50 µL/s.
Analysis of Soil Samples
A methanolic extraction technique was adapted from GC–MS for determination of BTEX in soil (7).
In the first step, linear detection of BTEX in the presence of high methanol concentrations was demonstrated over a range of BTEX concentrations (5–2000 ppbv in solution). Saturated sodium chloride solution (10 mL) was placed in 20-mL headspace vials. Standard solutions were prepared in methanol and 250-µL volumes of these were spiked into the aqueous solutions in sealed vials. Samples were incubated for 15 min at 60 °C. Then 2.5 mL of headspace was injected at 50 µL/s. Standard solution dilutions and spiking was carried out by the autosampler. Linearities as determined from R2 values-were greater than 0.997 for all compounds in the stated range and recoveries were 77–108% for 20–2000 ppbv solutions.
Next, soil samples were prepared as follows. Soil samples (2 g) were spiked with known amounts of primary standard and then sonicated with 6 mL of methanol (Merck, LiChrosolv grade), as extraction solvent, in 10-mL headspace vials using a Gerstel QuickMix shaker for 2 min. They were then centrifuged at 4500 rpm for 5 min using an Anatune CF200 centrifuge. A 250-µL aliquot of methanol was then taken and spiked into a 10-mL volume of saturated sodium chloride solution in a 20-mL headspace vial. Incubation and analysis by SIFT-MS were carried out using the procedure described above. Following the addition of the extraction solvent, the entire sample preparation and analysis was fully automated.
Analysis of BTEX Using SIFT-MS
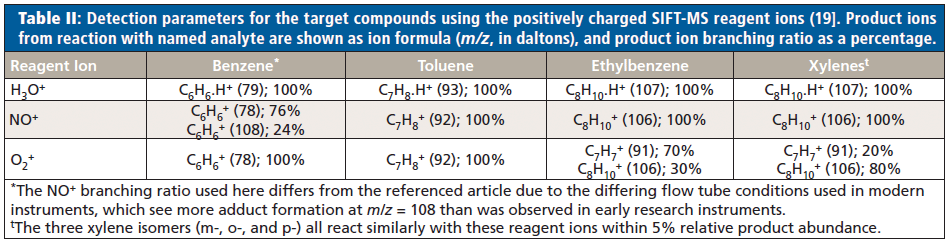
Parameters for detection of benzene, toluene, ethylbenzene, and the total xylene isomers using the positively charged reagent ions (H3O+, NO+, and O2+) of SIFT-MS are summarized in Table II. Rate coefficients are not shown, but these occur at or near collision rate for all reagent ions (19).
For ethylbenzene and the xylene isomers, the between-compound variability is less than 10% (that is, within experimental uncertainty), so that for H3O+ and NO+ the rate coefficients provide a valid sum of all C8H10 isomers. The NO+ reagent ion was used to quantify the total concentration of ethylbenzene and the three xylene isomers. To differentiate ethylbenzene from the total xylene isomers, the O2+ reagent ion was used because of the different product ion branching behaviors. Separation of the isomers was achieved through calibration of the 91 to 106 ratios using 20:80, 50:50, and 80:20 ethylbenzene–xylene mixtures carried out at two nominal concentrations (150 ppbv and 10 ppmv). The calibration results are summarized in Figure 1, where the two points for a given ratio arise from ratio determinations at the two concentrations, showing excellent agreement. Notice the linear response and the y-axis intercepts for 0% and 100% agree very well with the branching ratios shown in Table II for xylene and ethylbenzene, respectively (19).
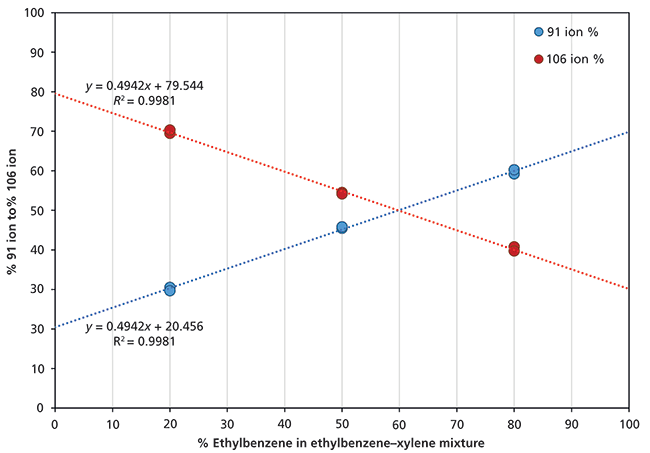
Figure 1: Calibration of relative abundance ratios (expressed as percentages) for the m/z 91 and 106 product ions formed when O2+ reacts with different mixtures of ethylbenzene and the xylenes. More details are given in the text.
Results and Discussion
Direct Air Analysis
Continuous air analysis demonstrates the speed of BTEX analysis using SIFT-MS. For a semicontrolled environment, the Anatune Limited laboratory proved ideal. This laboratory is used actively for demonstration and application development activities, both for conventional chromatographic methods as well as SIFT-MS, so the air is a relatively complex matrix of trace analytes (typical concentrations in the tens to hundreds of ppbv).
With the SIFT-MS instrument analyzing the laboratory air continuously (a measurement for each compound being acquired approximately every 3 s), various combinations of m-xylene and ethylbenzene (pure samples of each, and a mixture) were introduced from 20-mL headspace vials. Vials were shaken and then uncapped and recapped after a few minutes. After a few minutes, the laboratory windows were opened to ventilate the room before the next sample was opened.
Figure 2 shows the results obtained for ethylbenzene and m-xylene. The total concentration of ethylbenzene and m-xylene was measured using the NO+ reagent ion, whereas speciated ethylbenzene and xylenes concentrations were obtained using O2+. It is clear that the first exposure was xylenes, followed by ethylbenzene and then a 50:50 mixture of the two. Note that an additional 11 compounds (including benzene and toluene) were monitored concurrently, but these are not shown here for clarity.
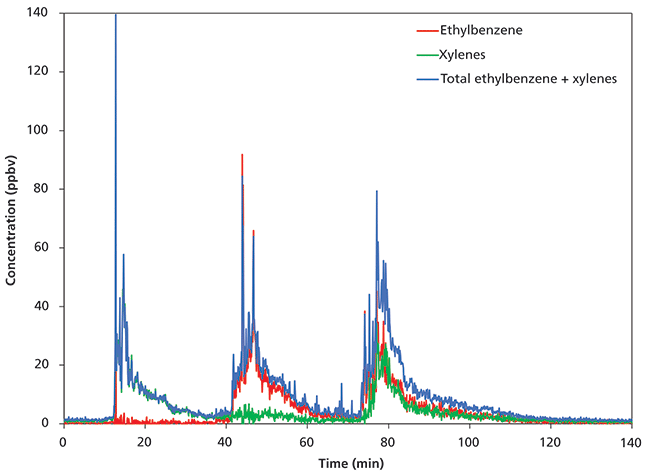
Figure 2: Separation of ethylbenzene and xylenes concentrations using O2+ with the total concentration (ethylbenzene + xylenes) superimposed.
Water Headspace
The high robustness of SIFT-MS analysis to humidity enables the technique to be applied to direct headspace analysis of BTEX and other hydrophobic volatiles (for example, chloroform) without any need for water stripping (that is, no purging, trapping, and drying).
Linear detection of BTEX and chloroform in the headspace of standard aqueous solutions (2.5–1000 ppbv in solution) is shown in Figure 3. Note that these data were generated without using an internal standard and exhibit excellent repeatability (Table III), with relative standard deviations (RSDs) better than 4% for solutions prepared at 250-nL/L (250 ppbv) concentrations. For this method, the limit of detection (LOD) is 0.3 nL/L and the limit of quantitation (LOQ) is 1 nL/L. These values are equivalent to an LOD of 0.26 µg/L for BTEX and 0.45 µg/L chloroform and an LOQ of 0.87 µg/L for BTEX and 1.5 µg/L chloroform.
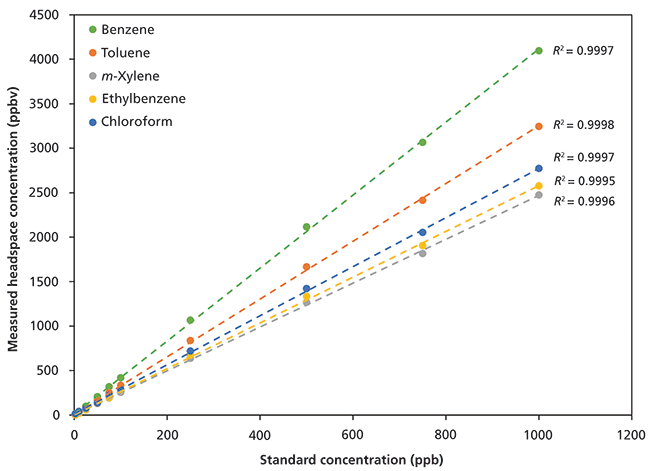
Figure 3: Linear detection of benzene, toluene, ethylbenzene, m-xylene, and chloroform from 2.5 to 1000 ppbv by volume in solution.
Ethylbenzene and the xylenes are very effectively differentiated in these headspace measurements (Figure 3). Notice that the ethylbenzene headspace concentrations are slightly higher than those of m-xylene, despite the standard being a 50:50 mixture. The higher benzene headspace concentrations result from a combination of water solubility and boiling point (benzene: 1.8 g/L, bp = 80 °C; toluene: 0.5 g/L, bp = 110 °C; ethylbenzene: 0.15 g/L, bp = 136 °C; m-xylene: 0.16 g/L, bp = 139 °C; chloroform: 8 g/L, bp = 60 °C). The order of BTEX compounds is therefore exactly as predicted on the basis of their physicochemical properties, whereas the position of chloroform arises because of its higher water affinity, despite its lower boiling point.
With current automation technology, which was optimized for GC–MS, we see a 3.5-fold increase in sample throughput when compared to the standard headspace GC–MS methods.
Soil Headspace
Significant levels of BTEX compounds can be found in soil because of pollution events-gasoline spills and leakage of underground storage tanks are some common causes. Various extraction approaches have been tried in the past, such as vapor partitioning. However, methanolic extractions are far more robust for extraction and recovery of VOCs from soil (7).
Methanolic extraction can also be applied with SIFT-MS when the NO+ reagent ion can be used for detection of target compounds, because of the slow reaction rate for the NO+ + methanol reaction. This constraint arises because H3O+ and O2+ both react rapidly with methanol, whereas NO+ reacts much more slowly (over 100-fold slower). All of the BTEX compounds are sensitively detected with NO+ (see Table II); benzene has two product ions with NO+, whereas toluene and the sum of ethylbenzene and the xylenes have one product ion. Because O2+ cannot be used with methanolic extraction, only a total concentration of ethylbenzene plus xylenes can be achieved.
Results for SIFT-MS headspace analysis following automated methanolic extraction of BTEX from spiked soil samples are shown in Figure 4. Linear detection is achieved using SIFT-MS, even in the presence of over 1000 ppmv of methanol in the headspace. Note that, for clarity, Figure 4 is plotted in terms of the spiked individual concentrations of ethylbenzene and m-xylene, but only the total is measured here because of residual methanol overwhelming the O2+ reagent ion signal, so speciation cannot be achieved.
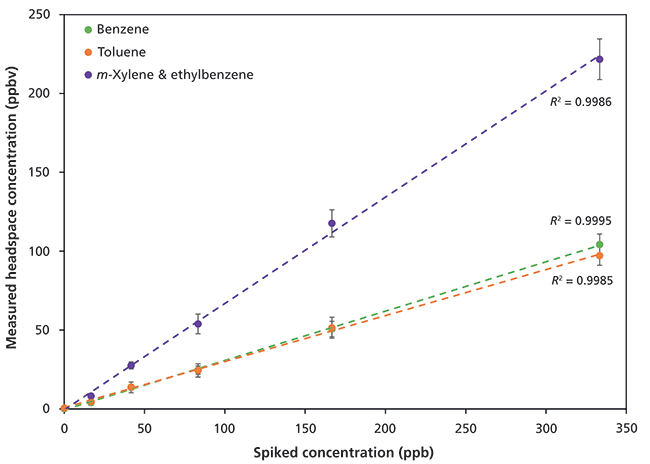
Figure 4: Linear detection of benzene, toluene, and the sum of ethylbenzene and the xylenes in spiked soil over the range 16-340 ppb following methanolic extraction. See the text for more details.
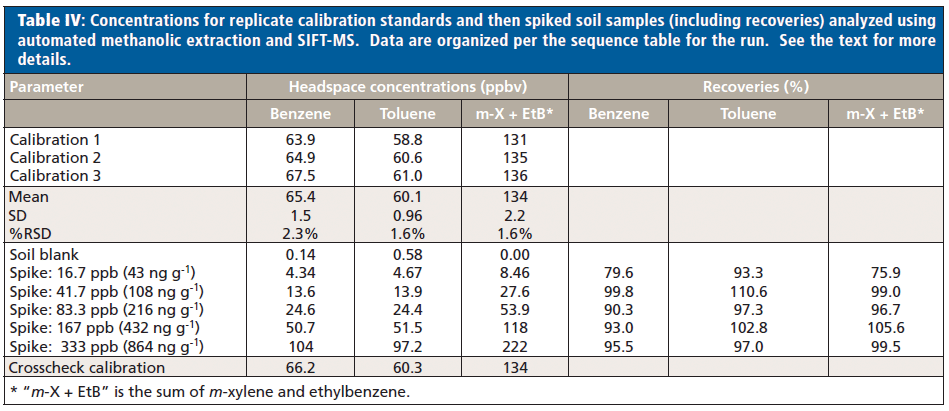
Table IV shows the results obtained for calibration standards and the various spiked soil samples in the sequence that they were run. Spike levels in parts per billion are applicable to all samples; the indicative mass per gram is calculated for each compound. RSDs for the calibrations are excellent and recoveries of 75–111% are all acceptable. For the experimental conditions used here, the LOQ and LOD are 40 ng/g and 12 ng/g, respectively. There is potential to achieve lower LOQs and LODs through better optimized extraction conditions.
This study has achieved a twofold increase in sample throughput for SIFT-MS when compared with GC–MS analysis using the current sequential sample preparation and analysis method. It may be possible to further increase this by using the multiple positions available with the autosampler modules and by batch-preparing the samples.
Conclusions
Monitoring isomers of aromatic hydrocarbons at low concentrations has traditionally been the domain of chromatographic methods to first achieve separation between the isomers followed by flame ionization or mass spectrometric detection. We have shown in this work that the DMS technique of SIFT-MS has enabled excellent differentiation of the ethylbenzene isomer from the xylene isomers using known O2+ reagent ion chemistry for both air and water. This approach can be applied in real time and in high-throughput applications both in testing and contract laboratories through to continuous process monitoring applications.
Future work will include fully validation of air, aqueous, and soil methods over the full linear range accessible to the SIFT-MS technique. It will also investigate multiple headspace extraction (MHE) as a means to directly analyze volatiles from soil without solvent extraction, thus overcoming the current impediment to resolving ethylbenzene and xylenes: methanol residue consuming the reagent ion.
References
(1) K. Morimoto and S. Wolf, Cancer Res. 40, 1189–1193 (1980).
(2) C.S. Chen, Y.C. Hseu, S.H. Liang, J.-Y. Kuo, and S.C. Chen, J. Hazardous Materials 153, 351–356 (2008).
(3) J.H. Suh, H.V. Lee, U. Kim, H.Y. Eom, J. Kim, H.-D. Cho, and S.B. Han, J. Separation Sci. 38(24), 4276–4285 (2015).
(4) M.A. Medinsky, P.M. Schlosser, and J.A. Bond, Environ. Health Perspect. 102, 119–124 (1994).
(5) R. Snyder, G. Witz, and B.D. Goldstein, Environ. Health Perspec. 100, 293–306 (1993).
(6) D.E. Chapman, T.J. Moore, S.R. Michener, and G. Powis, Drug Metab. Disposition 18(6), 929–936 (1990).
(7) A.D. Hewitt, Environ. Sci. Technol. 32, 143–149 (1998).
(8) C.-W. Tsao, H.-G. Song, and R. Bartha, Appl. Environment. Microbiol. 54(12), 4924–4929 (1998).
(9) United States Environmental Protection Agency. Compendium of methods for the determination of toxic organic compounds in ambient air using active sampling on to sorbent tubes. Compendium method TO-17. 1999. US Environmental Protection Agency, Cincinnati, Ohio.
(10) United States Environmental Protection Agency. Compendium of methods for the determination of toxic organic compounds in ambient air. 2nd edition. Compendium method TO-15. 1999. Cincinnati, Ohio.
(11) United States Environmental Protection Agency. Volatile organic compounds in water by purge and trap capillary column gas chromatography with photoionization and electrolytic conductivity detectors in series. Method 502.2. 1999. Munch, J.W. (Ed). Cincinnati, Ohio.
(12) T. Nilsson, F. Pelusio, L. Montanarella, B. Larsen, S. Facchetti, and J.O. Madsen, J. Sep. Sci. 18, 617–624 (1995).
(13) See the NIOSH International Chemical Safety Cards (https://www.cdc.gov/niosh/ipcsneng/nengnameA.html; accessed on December 5, 2017).
(14) P. Spanel and D. Smith, Med. & Biol. Eng. & Comput. 24, 409–419 (1996).
(15) D. Smith and P. Spanel, Mass Spec. Rev. 24, 661–700 (2005).
(16) B.J. Prince, D.B. Milligan, and M.J. McEwan, Rapid Commun. Mass Spectrom. 24, 1763–1769 (2010).
(17) V.S. Langford, I. Graves, and M.J. McEwan, Rapid Commun. Mass Spectrom. 28, 10–18 (2014).
(18) D. Hera, V.S. Langford, M.J. McEwan, and T.I. McKellar, Environments4, 16 (2017).
(19) P. Spanel and D. Smith, Int. J Mass Spectrom. 181, 1 (1998).
Mark J. Perkins is with Anatune Limited, Cambridge, United Kingdom. Vaughan S. Langford is with Syft Technologies Limited, Christchurch, New Zealand. Murray J. McEwan is with Syft Technologies Limited, Christchurch, New Zealand and the Department of Chemistry at the University of Canterbury, Christchurch, New Zealand. Direct correspondence about this article to: Vaughan.Langford@syft.com

New Study Shows FT-MIR Spectroscopy Can Authenticate Parmigiano Reggiano Farming Practices
March 11th 2025A new study published in the Journal of Dairy Science demonstrates that FT-MIR spectroscopy can effectively authenticate farming practices and dairy systems in Parmigiano Reggiano production but has limited ability to verify animal welfare parameters.
Advancing NIR and Imaging Spectroscopy in Food and Bioanalysis
March 11th 2025Our full-length interview with Huck covers more than just NIR spectroscopy in food and bio analysis. Spectroscopy sat down with Huck to also discuss current trends going on in spectroscopy, delving into what challenges spectroscopists face today and how they can solve these concerns.