The Humble Instrument Logbook?
Spectroscopy
Good practice (GxP) regulations require an instrument maintenance and use log. Have spectroscopy laboratories and software suppliers emerged from the Middle Ages yet?
When I walk around laboratories as an auditor, I usually stop at an instrument and check to see that it has an identity number, that it has been qualified, and that there is a calibration sticker on it. Then down the side of the instrument I often notice a scruffy, dog-eared, chemically stained book that looks as if it should be residing in a museum or the waste bin. Then the truth slowly dawns-it's the instrument logbook!
This column is dedicated to a discussion about the humble logbook for analytical instruments. Why do we need one and what needs to be entered in it? Finally, we'll look at why we can't automate the functions of a logbook. If we have complex computer-controlled spectrometers, why can't we dispense with the quill pen and cellulose and have an automated function that logs all work generated by the data system?
Why Do We Need an Instrument Logbook?
This is a good question to begin with: Why do we need any instrument logbook? One answer is required by either the good laboratory practice (GLP) or good manufacturing practice (GMP) regulations. Another answer is that it provides a history of the instrument, including information about the following events:
- use of the instrument,
- calibration to demonstrate that the instrument is working correctly,
- preventative maintenance visits from a service provider (or in some cases the angel of death),
- repairs and maintenance carried out in-house or by a service provider, and
- qualification and requalification of the instrument.
As such, a logbook is essential to demonstrate that an analytical instrument is under control, works correctly, and the results generated can be relied upon.
An instrument logbook is also one of the essential documents for data integrity. It is typically a bound and paginated paper book that is completed manually. It cannot be loose-leaf sheets or a ring-bound notebook purchased from a local supermarket, but instead must be a real book. You would be surprised by the number of laboratories that cannot get even this basic element right. The logbook is issued by a document control function, typically the quality assurance (QA) department, and when completed the log is returned to document control and then archived. To ensure that it is completed correctly, a written procedure must be available with training for making entries in an instrument logbook, and also for reviewing them. There needs to be a means of referencing the reports from service visits and any qualification of the instrument, and both need to be accommodated to ensure a complete record for each instrument. The other item that is often neglected is who is going to review the entries and when.
What Needs to Be Entered in the Logbook?
Since we all work to the same regulations, you would think that the contents of an instrument logbook would be easy and straightforward. Unfortunately, optimism falls at the first hurdle. We use the same regulations, but everyone interprets the words differently. At this point it is important to remember one of the corollaries of Murphy's Law-Cahn's Axiom-when all else fails read the regulations. Table I lists the GLP and GMP requirements for an instrument maintenance and use log.
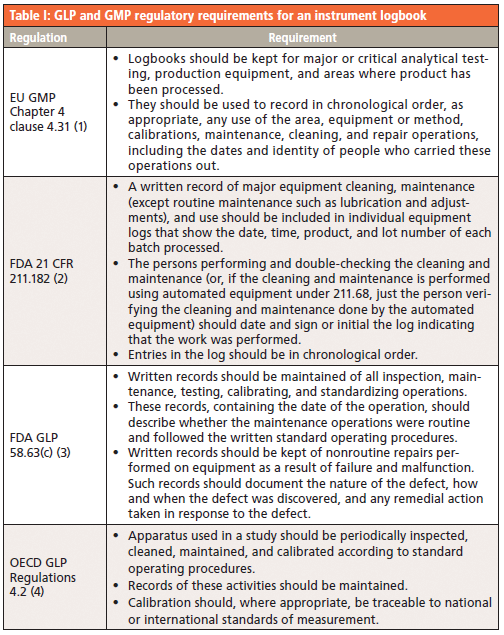
Summarizing these regulations, the logbook entries must be
- written;
- linked to the use of the instrument (that is, they should include information about practices such as use, calibration, maintenance, and so forth);
- chronological, which implies that the entries must be dated and must be made contemporaneously in the sequence they are performed on a day-the best advice is do not separate the tasks of use, calibration, and so forth, but list them all chronologically;
- checks for correct operation of the instrument; and
- a description of the analytical work undertaken by the instrument.
In addition to the elements listed above, you also need to consider any pharmacopoeia requirements. For example, pharmacopoeia requirements could include system suitability test results or sound science such as point of use checks made on the day to show that the system is working correctly (such as calibration of a balance or pH meter or running a standard spectrum for a spectrometer).
You would think that from this list it would be simple, right? Wrong! How will use of the instrument be interpreted? Does each use mean just the work package performed or individual runs within that package? It depends on the interpretation by an individual organization of the regulations. But, we now have additional requirements for the logbook that potentially cloud the issue:
- The Food and Drug Administration's (FDA) Part 11 Scope and Application Guidance (5) allows laboratories to record audit trail information if an application does not have such functionality.
- Data integrity requirements mean that the logbook takes on an increased importance for recording the work, potentially resulting in increased granularity for the entries. This increased level of detail may include verification by a second person of an instrument reading where output is not captured electronically or by an attached printer. Or entries in lieu of an audit trail for corrections to data.
And yes, you've guessed it-all entries are handwritten!
The importance of the instrument logbook has been known by regulatory agencies for a long time. Enter stage left my North American advertising agency . . . the FDA.
FDA Guidance on Inspection of Pharmaceutical Quality Control Laboratories
The FDA guidance for inspectors on the "Inspection of Pharmaceutical QC Laboratories" was issued in 1993, following the Barr Laboratories case, and has an interesting approach that is also very useful for second-person reviews and data integrity audits (6). The guidance states "Laboratory records and logs represent a vital source of information that allows a complete overview of the technical ability of the staff and of overall quality control procedures."
Note the three key phrases:
- vital source of information,
- technical ability of staff, and
- overall quality control (QC) procedures.
Why does the FDA think this about a humble instrument logbook? The reason is that a logbook is not humble, but a vital link between the analytical instrument, the data system, and the work carried out as shown in Figure 1. The logbook links the instrument or spectrometer system with the work performed by or on the instrument and the data system, such as qualification and requalification work, method development, method validation, calibration checks, system suitability tests, routine work, repairs, and preventative maintenance. The aim is to have a complete record to identify issues and aid in troubleshooting issues. Data from some system suitability test or point of use checks should be trended to identify any instrument or method issues that could result in an out-of-specification result. A few laboratories do this via the instrument logbook for simple instruments such as balances and pH meters. However, this is an area that should be automated by the instrument data system and linked to an automated logbook.
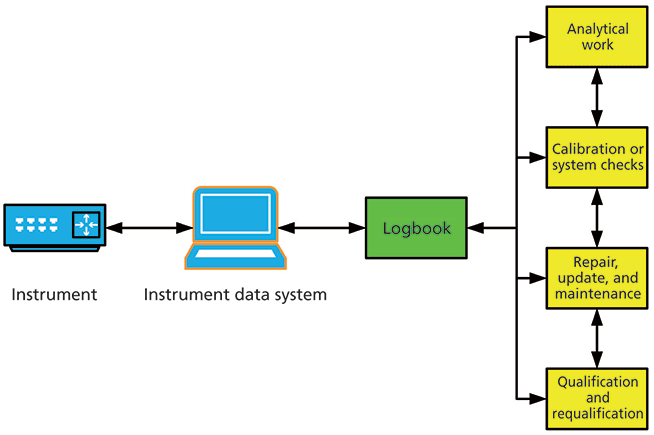
Figure 1: Relationship between the instrument logbook, the analytical instrument, and the work performed.
Now let's compare the theory with the practice as found in inspections.
483 Observations for Laboratory Logbooks
In May 2016, Akorn AG received a 483 citation for discrepancies in the sample logbook entries used to document storage conditions as either ambient or 2–8 °C (7):
- Between February 17 and May 18 there were 24 instances where a single sample was noted as being stored at both temperatures, so it was not known if samples were correctly stored before analysis.
- Write-overs of entries where dashes were changed to X's
- Review of the logbook by a supervisor only consisted of checks for empty spaces. Discrepant entries (such as write overs) had not been identified or investigated for their impact on stored samples or the corresponding analytical data.
The Aarti warning letter of July 2013 included the following citation (8):
- On October 27, 2012, our investigator noticed that a QC analyst was performing a loss on drying (LOD) analysis for <redacted> and had recorded the completion time as <redacted> and total time as <redacted> in the usage logbook for the LOD oven usage logbook although the step was not yet completed.
How do these citations compare with the criteria identified in a quarter of a century-old FDA guidance document? Consider the following questions:
- What do you think of the technical ability of the staff (or more accurately, the inability of the staff)?
- Are the written procedures adequate or even followed?
- If you were an auditor or an inspector, what would be your view of the technical competence of this laboratory?
Just from these two citations you can start to see the importance of a correctly completed and reviewed instrument logbook.
Instrument Logbooks in Practice
Typically, the majority of instrument logbooks are paper based using bound books with sequentially numbered pages to prevent pages from being removed and replaced. You would be surprised that I still find instrument logbooks that fail to meet these simple requirements-bound but without numbered pages, books that have loose leaf pages, or ring-bound books from which pages can be torn out without leaving a record of what they contained.
An instrument logbook requires all users to complete the entries manually, consistently, and contemporaneously. The way the use of an instrument is recorded will depend on the interpretation of the regulations in Table I by each individual organization. However, because the logbook is paper it is always difficult to know if an entry has been made contemporaneously or before or after the event.
As an auditor, one item that is usually overlooked is that each instrument logbook in any analysis should be subject to second-person review to ensure that the work is correctly documented. As can be seen in the 483 observations earlier, some reviews can be superficial and ineffective. The second-person review may look at a single entry or connected entries, but there is also the need for the instrument owner to check all entries periodically to confirm that over time the instrument is operating correctly. What do you mean you don't have an instrument owner? Get one appointed-now!
Role of the Instrument Logbook in the Second-Person Review
One of the important stages of analysis is the second-person review-the third and fourth eyes of the four eyes principle. Primarily, it is good science to have a check of results to ensure that procedures have been followed, samples have been analyzed correctly, data have been interpreted correctly, calculations were right, and the reportable result was accurate. Increasingly, the second-person review is a means of ensuring data integrity and preventing poor data management practices or falsification of data. An outline of a second-person review is shown in Figure 2. Here there are three key sources of information for the reviewer: paper printouts, electronic records in the data system, and the instrument logbook. The reviewer needs to check all three to see that records are complete, consistent, and accurate and that the three are congruent and there are no discrepancies. Omissions or discrepancies with anything between the three parts need to be investigated thoroughly: Is a discrepancy the result of a mistake or is it something more sinister?
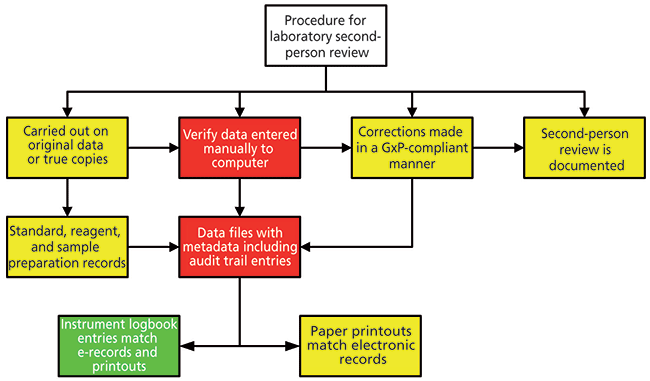
Figure 2: Role of the instrument log in a second-person review.
Is an Instrument Performing OK?
An instrument logbook is often a repository of data about the day-to-day performance against a reference or calibration standard. Over time it can tell you if an instrument is continuing to perform acceptably. However, because this information is stored on paper there is often little attempt made to trend the data to see if there is a potential problem and stop it from getting worse either though service or repair. This oversight is a shame because often out-of-specification situations could be mitigated or even prevented if these data were plotted and trended-even if only the results were visualized over time against acceptance and warning limits. This is one of the roles of the instrument owner-to confirm that the instrument is working as expected. In addition, the individual may also be responsible for coordinating service and repair of the instrument.
Automating the Instrument Logbook
When we move our attention to laboratory computerized systems we return to the medieval age. We have the ridiculous situation where a lot of money has been spent on a spectrometer with great resolution complete with (mostly) an amazing software application to control it. What about the instrument logbook? Paper! The application software has the sequence file, the work performed, a record of the samples, the analysis method, and so forth, but what does the poor analyst have to do? Write all this information into the instrument logbook complete with typos and mistakes. What an amazing waste of time and effort when the information-in probably more detail than is required by local procedures-already exists in the instrument data system.
Why can't we have an electronic instrument logbook that is generated automatically by the instrument control software? It would be protected and secure like an audit trail and could be viewed as part of a second-person review with a function to document when selected entries have been reviewed. The instrument log function could not be turned off, which like an audit trail will result in a true record of the work performed by and on the instrument. The log would be an additional source of information to ensure the integrity of the work performed by an instrument. In addition, there should be the ability to cross-reference between the audit trail and logbook entries and drill down into the underlying data and metadata.
Qualification and maintenance work may be more difficult to document, but this challenge should be surmountable by instrument companies because many instrument data systems can identify an instrument down to the serial number. Therefore, some linkage between qualification work and the online instrument logbook is also required, and that linkage should extend to any paper records of service visits outside of the log. This may be done via a controlled user input to the instrument log.
Summary
We have looked at the humble instrument logbook, which is really not so humble-it is a vital source of information about the instrument and the work undertaken by it. Unfortunately, virtually all logbooks are based on paper and completed manually, which is a shame when all the information available in an instrument data system could be abstracted and used for an automated logbook.
Is this concept too simple, and you still want the comfort of your cellulose instrument logbooks? For those that don't, what are you going to do about it? Will you suffer in silence or start to request this functionality from the suppliers of your software? The reason you don't have this functionality is that suppliers are market driven-if the market does not require it then nothing happens. Over to you . . .
Acknowledgments
I would like to thank Kevin Roberson and Mark Newton for helpful comments made in the preparation of this column.
References
(1) European Commission Health and Consumers Directorate-General, EudraLex: The Rules Governing Medicinal Products in the European Union. Volume 4, Good Manufac turing Prac t ice Medicinal Products for Human and Veterinary Use. Chapter 4 Documentation (Brussels, Belgium, 2011).
(2) Code of Federal Regulations (CFR), 21 CFR 211 "Current Good Manufacturing Practice for Finished Pharmaceutical Products" (Food and Drug Administration, Sliver Spring, Maryland, 2008).
(3) Code of Federal Regulations (CFR), 21 CFR 58 "Good Laboratory Practice for Non-Clinical Laboratory Studies" (Food and Drug Administration, Washington, D.C., 1978).
(4) Organsation for Economic Co-operation and Development (OECD), Series on Principles of Good Laboratory Practice and Compliance Monitoring Number 1 (OECD Principles on Good Laboratory Practice, Paris, France, 1998).
(5) U.S. Food and Drug Administration, Guidance for Industry, Part 11 Scope and Application (FDA, Rockville, Maryland, 2003).
(6) U.S. Food and Drug Administration, Inspection of Pharmaceutical Qualiy Control Laboratories (FDA, Rockville, Maryland, 1993).
(7) U.S. Food and Drug Administration, "Form 483 for Akorn AG Switzerland" (FDA, Silver Spring, Maryland, 2016).
(8) U.S. Food and Drug Administration, "Warning Letter Aarti Drugs Limited" (FDA, Silver Spring, Maryland, 2013).
R.D. McDowall

R.D. McDowall is the director of R.D. McDowall Limited and the editor of the "Questions of Quality" column for LCGC Europe, Spectroscopy's sister magazine. Direct correspondence to: SpectroscopyEdit@UBM.com
