Observing the Solar Spectrum at House of Science
Application Notebook
Analysis of the temperature of the photosphere and determine the temperature by students using CCD spectrometers.
K.E. Johansson1, C. Kozma2, and Ch. Nilsson2, 1Stockholm House of Science and Department of Physics, Stockholm University and 2 Department of Astronomy, Stockholm University
Analysis of the temperature of the photosphere and determine the temperature by students using CCD spectrometers.
The main process responsible for the energy production in the Sun is the proton-proton chain reactions. In these reactions, four hydrogen nuclei are fused into a helium nucleus. The sum of the masses of the four hydrogen nuclei is slightly larger than the mass of the final helium nucleus, and this mass is turned into energy described by Einstein's equation, E = mc2 . The mass difference between four protons and one helium nucleus, 4.6 10-29 kg, is transformed into 27 MeV of energy including the annihilation of the two positrons created in the process. Each second about 4 million tonnes of matter is converted into 2 1039 MeV of energy in the Sun, most of it in the form of gamma-rays.
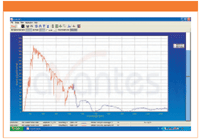
Figure 1: Solar spectrum.
Experimental Details
House of Science has six CCD spectrometers Avantes AvaSpec-2048 from Azpect Photonics, with which the radiation from the Sun is studied. The CCD has an array of 2048 pixels and covers the wavelength range 200–1100 nm. The observed sunlight comes from a thin layer of the solar atmosphere, the photosphere. The thickness of the photosphere is only 400 km which is very thin compared to the 700,000 km radius of the Sun.
Spectrum Analysis
Blackbody Radiation
Sun radiates almost as a blackbody, that is the radiation spectrum is determined by the temperature. Using Wien's displacement law:
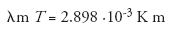
where λm is the wavelength at which the Planck curve has its maximum, it is possible to determine the temperature (T) of the photosphere. From their observations, the students estimate the value of λm either by visual inspection or with the help of the AvaSoft software which comes with the CCD spectrometers. Usually, the students find a value of the temperature of around 5800 K.
Absorption Lines
There are several absorption lines superimposed on the blackbody spectrum. These lines originate from the upper parts of the photosphere. By determining the wavelength for these lines it is possible to identify the elements in the solar atmosphere that are causing them. It is important to go through the energy level diagram of hydrogen and how absorption lines arise, particularly the Balmer lines emerging from transitions from the second energy level in the hydrogen atom. The Balmer lines are emitted in the optical region of the spectrum and are detectable with the spectrometer. The students identify them in their observed spectrum and also can identify lines from other elements in the solar atmosphere like calcium, magnesium, and iron. The sunlight also passes through the Earth's atmosphere and strong absorption lines from H2O and O2 molecules in the Earth's atmosphere can be seen.
Emission Lines
As a complement to the solar observations, the students study the spectrum from a hydrogen lamp. This spectrum is an emission spectrum containing both hydrogen and oxygen lines. The difference between absorption and emission lines is discussed with the students. They easily identify at least six components of the Balmer series. By accurately determining the wavelengths of the Balmer lines (quantum numbers n = 2 and m = 3,4,5..) with the spectrometer software the students are able to calculate Rydberg's constant (RH) from Bohr's formula:
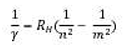
Avantes BV
Oude Apeldoornseweg 28, 7333 NS Apeldoorn, The Netherlands
tel. +31 313 670 170; Fax: +31 313 670 179
Email: info@avantes.com; Website: www.avantes.com
