Online Identification of Flavones from Flos Chrysanthemi by LC–MS-IT-TOF
Special Issues
The use of medicinal herbs as alternative treatment methods continues to grow. With this escalating use has come an increasing interest in determining the chemical compositions of these herbs in order to obtain a better understanding of their makeup and effects. In this study, Flos Chrysanthemi, a commonly used traditional Chinese medicine that has been cultivated for centuries, was analyzed to identify the main flavone compositions in one original breed of Flos Chrysanthemi (Hangbaiju) in China.
As an alternative treatment method, medicinal herbs have attracted significant attention. Many are proving to be safe and effective. In recent years, there has been an increase in their use. For example, in 2004, one quarter of adults reported using an herb to treat a medical illness within the previous year (1).
Flos Chrysanthemi is a commonly used traditional Chinese medicine with diverse activity, such as antibacterial, antifungal, antiviral, anti-inflammatory, and antioxidant activities. It also has been reported to reduce risks of certain cancers and cardiovascular diseases. According to previous chemical studies, the main chemical compositions of Flos Chrysanthemi are essential oil, flavonoids, caffeotannic acid, amino acids, and microelements.
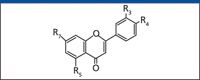
Flos Chrysanthemi has been cultivated for centuries, and there are numerous breeds, each of them possessing different chemical constituents. No systematic research concerning its chemical compositions has been reported previously. The aim of this study was to identify the main flavone compositions in one original breed of Flos Chrysanthemi (Hangbaiju) in China.
Mass spectrometry (MS) has begun to play an increasingly important role in the field of natural product chemistry. Compared to other kinds of mass spectrometers, an ion trap combined with a time-of-flight mass spectrometer allows high-precision and rapid MSn experiments. It can provide precise formulas of product ions directly using both high accurate mass analysis and multistage mass fragmentation.
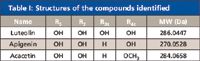
Table I: Structures of the compounds identified
In addition, until recently, UV data often were not utilized in identification work, as they were thought only important for quantitative analysis. However, the addition of UV shift reagents induces a displacement of the absorption maxima that can be used to determine the positions of the free hydroxyl groups of flavone.
Therefore, a liquid chromatography (LC)–UV (postcolumn derivatization) electrospray ionization (ESI)-MSn method for rapid and exact analysis of chemical components found in herbal medicine was developed and applied for the identification and determination of flavones in Flos Chrysanthemi.
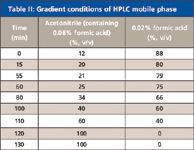
Table II: Gradient conditions of HPLC mobile phase
HPLC–UV Analysis
UV analysis of the methanolic extract of Flos Chrysanthemi was performed on a Shimadzu (Kyoto, Japan) Prominence LC-20A liquid chromatography system with photodiode-array (PDA) detection. The separation was achieved on a YMC C18 column (150 mm × 4.6 mm, 5 μm) combined with a Diamonsil C18 column (250 mm × 4.6 mm, 5 μm). The components were eluted with a linear gradient of acetonitrile–water (containing 0.02% formic acid in positive ion mode), shown in Table II. The oven temperature was 40 °C, the flow rate was 1.0 mL/min, and the UV detection wavelength was 335 nm. UV spectra were recorded between 190 and 400 nm.

Table III: Experimental conditions for the post-column addition of UV shift reagents
HPLC–MSn Analysis
High performance liquid chromatography (HPLC)–MSn was performed directly after UV measurements. A Shimadzu LC–MS-IT-TOF mass spectrometer with an ESI interface was used with the following conditions: interface voltage and current were 4.50 kV and 1.6 μA for positive ion mode, and –3.50 kV and 1.7 μA for negative ion mode, respectively. Nebulizing gas was 1.5 L/min. Curved desolvation line (CDL) and heat block temperature were both 200 °C. MSn experiments were performed by programming dependent scan events. All of the relative energy in collisions was 50%. Detector voltage of the time-of-flight (TOF) analyzer was 1.70 kV. Ultrahigh-purity argon was used as the collision gas for collision-induced dissociation (CID) experiments.
Mass calibration was carried out using a trifluoroacetic acid sodium solution (2.5 mmol/L) from 50 to 1000 Da. Data acquisition and processing were performed using LCMSsolution software supplied with the instrument. The ion accumulation time for each precursor ion was 50 ms.

Figure 1: Instrument flow diagram for post-column derivatization analysis.
The method used for postcolumn addition of UV shift reagents was based upon a previously reported protocol (2). For flavone analysis, the following reagents were used: at room temperature, sodium acetate (weak base), sodium hydroxide (strong base), and sodium acetate–boric acid; and at 90 °C (to ensure full derivatization), aluminum chloride under acidic and nonacidic conditions.
LC–UV (Postcolumn Derivatization) ESI-MSn Analytical Results
With the optimized parameters, MSn (n ≤ 5) data were obtained in both positive and negative ion modes, providing prolific structural information. A total of 25 compounds in the Flos Chrysanthemi HPLC fingerprint were identified and determined. A total of 18 compounds presented typical UV spectra of flavones. Identified flavones with online UV and MS structural information are given in Table IV, in which the 18 identified flavones are divided into four groups.
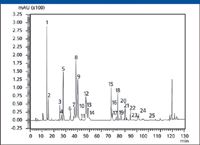
Figure 2: HPLCâPDA chromatogram of a methanolic extract of Flos Chrysanthemi (Hangbaiju).
Based upon the (±)ESI-MSn data, UV shift information, the standards, and many related references, the structures of 18 flavones were identified.
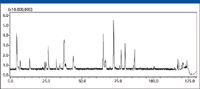
Figure 3: The total ion chromatogram of a metholic extract of Flos Chrysanthemi (Hangbaiju) in positive ion mode.
Novel Aspects
It has been widely accepted that the medicinal effects of herbal medicines are dependent upon the interaction of their multiple constituents. The HPLC fingerprint technique is used as a quality control method to ensure the consistency of herbal medicines. However, the HPLC fingerprint can only provide information on peak retention time without information about chemical structures. Even when chemical standards were used in this kind of fingerprint analysis, identification results were obtained based upon retention times, which are not always conclusive.

Table IV: Online UV and MS(+) structural information of a methanolic extract of Flos Chrysanthemi
Therefore, the LC–UV–ESI-MSn method to identify and determine the constituents in herbal medicines was developed. This method can provide multiple and accurate chemical structural information based upon high-precision data and multiple-stage mass fragment information. In addition, the application of online postcolumn derivatization helps to confirm exact positions of free hydroxyl groups in flavones, which guarantees the accuracy of identified results. Also, it is a facile method to obtain the UV shift results of numerous constituents simultaneously. As a result, the application of the LC–UV–MSn technique with online postcolumn derivatization will increase the reliability and accuracy of quality control work and also improve work efficiency.

Table V: LCâMSn data of standards; data from the Flos Chrysanthemi HPLC fingerprint
Additionally, with the multiple structural information acquired, it can become a guide for chemistry study in herbal medicine and solve numerous problems in this area.
Liangliang Yin and Shizhong Chen are with Peking University, School of Pharmaceutical Science, Beijing 100191, China. Jing Dong and Hashi Yuki are with Shimadzu Corporation/Global COE, Application & Technical Development, Kyoto, Japan.
References
(1) S. Ben, and R. Ko, Am. J. Med. 116(7), 478–485 (2004).
(2) J.L. Wolfender and K. Hostettmann, J. Chromatogr. 647, 191 (1993).

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.