OPCO Laboratory Inc.
Company Description
OPCO Laboratory was founded over 30 years ago by a spectroscopist from Jarrell-Ash to serve the atomic spectroscopy community with ultraviolet coatings for gratings and mirrors. Today, with a full-time spectroscopist on staff, OPCO remains a leading provider of custom components to researchers and engineers, and a major supplier to many of the largest spectrometer manufacturers. OPCO is a fully integrated manufacturing operation with optical design services available, which is why we can take your sketch and ship your prototype quickly. Our proprietary replication process, clean room assembly areas, and metrology capabilities ensure we can manufacture and assemble cost effective solutions to your optical needs.
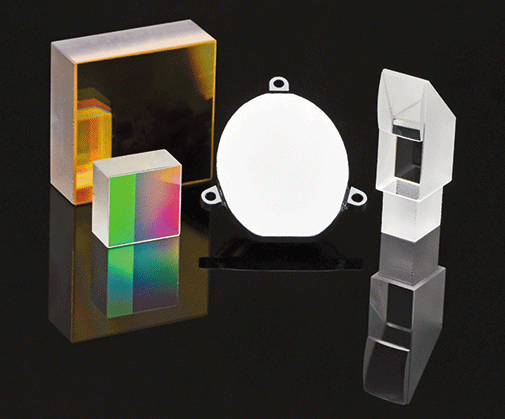
Chief Spectroscopic Techniques Supported
- Atomic spectroscopy (AA, ICP, arc/spark, LIBS)
- UV-vis-NIR
- IR and FT-IR
- Raman spectroscopy
- Spectrometer optics for space vehicles
Markets Served
OPCO Laboratory is a leading provider of custom optical components and assemblies for a wide range of scientific and industrial applications including: atomic and molecular spectroscopy, semiconductors, astronomy, high energy physics, medical devices, aerospace, and defense.
Major Products/Services
Spectrometer Optics
- Gratings
- Prisms, Windows and Lenses
- Mirrors (spherical/toroidal/flat)
Diffraction Gratings
- Custom Rulings
- Volume Replication
- Library of Existing Masters
Cost Effective Replication
- Metal, Glass, Ceramics
- Flats, Spheres, Toroids
- Integrated Mounting Features
Facility
OPCO Laboratory is located in Fitchburg, Massachusetts. Our vertically integrated facility includes equipment for cutting, shaping, and polishing glass; six coating chambers; an optics replication lab; and several assembly clean rooms.

OPCO Laboratory Inc.
704 River St.
Fitchburg, MA 01420
TELEPHONE
(978) 345-2522
FAX
(978) 345-5515
E-MAILsales@opcolab.com
WEB SITEwww.opcolab.com
YEAR FOUNDED
1982

New Multi-Spectroscopic System Enhances Cultural Heritage Analysis
April 2nd 2025A new study published in Talanta introduces SYSPECTRAL, a portable multi-spectroscopic system that can conduct non-invasive, in situ chemical analysis of cultural heritage materials by integrating LIBS, LIF, Raman, and reflectance spectroscopy into a single compact device.
Thermo Fisher Scientists Highlight the Latest Advances in Process Monitoring with Raman Spectroscopy
April 1st 2025In this exclusive Spectroscopy interview, John Richmond and Tom Dearing of Thermo Fisher Scientific discuss the company’s Raman technology and the latest trends for process monitoring across various applications.