Qualifying ATR Accessories for Pharmaceutical Applications
Application Notebook
Recent advances in single-bounce ATR accessories provide an easy way to acquire a high-quality infrared spectrum from very small amounts of sample in less than 1 min. This has resulted in increased use of this technique in the pharmaceutical industry, making accessory qualification a concern. We suggest an adaptation of the Pharmeuropa guidelines as an excellent starting point.
FT-IR spectrometry is a key technique used throughout the pharmaceutical industry and most of the pharmacopoeias in the world contain a section on how to qualify an infrared spectrometer for use. However, there is no clear guidance from regulatory agencies on how to qualify an FT-IR spectrometer system with an ATR accessory. In fact, there is some question whether this falls under “Analytical Instrument Qualification” or “System Suitability.” While the US Pharmacopeia defines appropriate measurements for wavelength accuracy for transmission spectroscopy, it clearly states that ATR spectra are different. The European Pharmacopoeia recommends the same polystyrene peak positions but adds a resolution test. A recent Pharmeuropa report (29.3) describes some proposed changes to the European Pharmacopoeia and actually proposes ATR peak positions for polystyrene as well as pointing out that a different resolution test is also necessary. One challenge with ATR spectra is the relative differences in peak intensity. While some of the peaks in a transmittance spectrum of 35 µm thick polystyrene film are highly absorbing and not appropriate for verifying wavelength accuracy, they are much weaker in an equivalent ATR spectrum, particularly in the C-H stretching region. In this application note, we will describe an evaluation method based on the Pharmeuropa proposal that we believe will verify the performance on an ATR accessory in FT-IR instruments.
Experimental Conditions
Spectra were acquired on various FT-IR spectrometers with different single-bounce ATR accessories at 2 cm-1 instrument resolution using a NIST traceable polystyrene standard that had been qualified in a calibration laboratory. A workflow was created using the four peak locations proposed for ATR qualification. We chose to use the full width at half maximum on polystyrene peaks at 1154 cm-1 and 906 cm-1 to determine spectral resolution. The instrument resolution was confirmed using water vapor peaks with the accessory mounted in the system. Figure 1 shows the spectra from a polystyrene film in transmission (blue) and the ATR spectrum (red) acquired with the diamond iD5 Accessory on a Thermo Scientific™ Nicolet™ iS™ 5 FT-IR spectrometer.
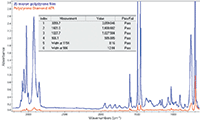
The results (targets and actual) of the proposed pharmacopoeia measurements are tabulated (inset) for the diamond ATR spectrum. While we obtained good results using the peaks described in the Pharmeuropa proposal, the peak near 3060 cm-1 is quite weak, a situation which would be even worse for Ge-ATR (shallower depth of penetration).
We conclude that the guidelines recommended by Pharmeuropa provide an excellent starting point for this long overdue development. However, we strongly recommend replacement of the 3059.7 cm-1 peak with either of the stronger signals near 3025 cm-1 or 2920 cm-1, which will provide a more robust measurement, regardless of the ATR crystal type.
Questions about this application note may be directed to: steve.lowry@thermofisher.com.

Thermo Fisher Scientific
5225 Verona Rd., Madison, WI 53711
tel. (800) 532-4752
Website: www.thermofisher.com
