Rapid Enantiodifferentation of Chiral Organophosphorus Compounds by 31P NMR Spectroscopy in the Presence of α-Cyclodextrin as the Chiral Solvating Agent
Spectroscopy
This method, for the evaluation of the enantiomeric purity of particular phosphonate derivatives, offers advantages in terms of cost, simplicity, and measurement speed.
Applications of α-cyclodextrin to the evaluation of the enantiomeric excess in mixtures of chiral phosphonate derivatives with one and two stereogenic centers with additional heteroatoms in their structures is presented. Two classes of compounds, α-aminophosphonic acids and α-hydroxyphosphonates, were separated into their respective enantiomers using chiral solvating agents at specified pH (pD) values, which is a crucial factor for the quality of enantiodiscrimination via 31P nuclear magnetic resonance (NMR) spectroscopy. The main advantage of such an approach is associated with the cost involved, the simplicity, and the speed of the purity determination.
Phosphonates are organophosphorus compounds that possess one P-C bond and tend to be very stable and resistant to both thermal or chemical degradation as well as biochemical decomposition. Derivatives of phosphonic acids have documented biological activity and are also of great industrial importance as water treatment chemicals, agents for metal processing, and fire retardants (1). Hydroxyphosphonates are an important subset of this group of phosphonate derivatives. They behave as enzyme inhibitors, antibacterial and antifungal agents, herbicides, and antitumor or antiviral drugs. They are also used as drugs in the treatment of malaria, AIDS, and cytomegalovirus infections (2–5). Additionally, α-aminophosphonic acids are analogs of the natural amino acids in which the carboxylic acid has been replaced by phosphonic acid moiety. These compounds may exhibit inhibitory activity, act as antibiotics, crop protection agents, or herbicides and peptide mimics (6–9). This wide range of possible applications is, in most cases, characteristic of chiral compounds with defined absolute configurations. That is why elaboration and perfection of methods for the assessment of enantiomeric excess and optical configuration represent important but, at the same time, difficult and challenging aspects. The work presented here focuses on the application of chiral solvating agents for the evaluation of the enantiomeric purity of particular phosphonate derivatives. These results represent a continuation of previous work devoted to applications of cyclodextrins (10). Thus, cyclodextrins (CDs) are divided into α-, β-, and γ-cyclodextrin. α-Cyclodextrins are cyclic oligosaccharides that consist of six residues of D-glucose units connected via α-1,4-glycosidic bonds. They possess a hydrophilic surface on the outside and a relatively hydrophobic cavity in the center. These differences suggest that cyclodextrins (hosts) have the ability to accept smaller compounds (guests) into their molecular cavity. This chiral solvating agent is very useful and universal for the analysis of the enantiomeric composition of compounds with single aryl or alkyl substituents (4,10,11).
The present paper describes an experimental protocol for the use of α-cyclodextrin as a chiral solvating agent, which is applied to the enantiomeric excess determination for a set of phosphonate-containing compounds. Enantiodifferentiation of organophosphorus compounds with α-cyclodextrin used as the chiral solvating agent is a simple, efficient, and versatile method that enables the determination of the enantiomeric purity via 31P nuclear magnetic resonance (NMR) spectroscopy recorded in a chiral environment. In the case of phosphonate derivatives, 31P NMR spectroscopy is applied for such a purpose, following the addition of chiral solvating agents or chiral derivatizing agents (CDAs) (4 9,10,12–14) and further avoids the use of 1H NMR, where suppression of the residual solvent (H2O in D2O) would be required and extensive overlap of the proton signals may be a potential problem.
The main goal of the present work was elaboration of a method that is useful for the determination of enantiomeric composition of derivatives of phosphonic acids (Figure 1) using 31P NMR spectroscopy with the use of added α-cyclodextrin as a chiral discriminator. The structures drawn in Figure 1 were very difficult experimental targets mainly because of their solubility (other requirements than cyclodextrin) and steric structure (for example, two stereogenic centers). The optimization of the method was performed taking into account the pH of the solution in D2O (pD) and the concentration of α-cyclodextrin (100 mM) in the samples.
Figure 1: The chemical structures of derivatives of phosphonic acids: α-aminophosphonic acids, compounds 1–3; α-hydroxyphosphonates, 4 and 5; and N-derivatives of α-aminophosphonic acids, 6–8.
Experimental
All chemicals were purchased from commercial suppliers: Fluka, Avantor Performance Materials Poland S.A., and Sigma Aldrich. Compound 2 was a kind gift from Professor Kafarski of the WrocÅaw University of Technology and University of Opole (16). Compound 3 was synthesized by Dr. Tomasz K. Olszewski of WrocÅaw University of Technology (15). NMR spectra were measured on a Bruker Avance 600 system at observation frequencies of 600.58 MHz for 1H, 243.12 MHz for 31P in D2O (99.9% of atom D), and 15,102 MHz for 13C NMR in D2O. Chemical shifts (δ) are expressed in parts per million (ppm) and coupling constants (J) are given in Hz. 1H NMR spectra are referenced to the central line of the solvent (δ = 4.78 for water). In the following section, the underlined letters indicate the protons involved in the analysis.
Synthesis of α-Aminophosphonates
1-Amino-2-methylbutanephosphonic acid (compound 1) was synthesized according to the method described in the literature (16). Pure compound 1 was obtained as a mixture of diastereomers in a molar ratio (A:B) of 1:0.45.
1-Amino-2-Methylbutanephosphonic Acid (Compound 1)
A: 31P NMR (D2O, δ, ppm): 13.22 1H NMR (D2O, δ, ppm): 0.93 (triplet, J = 7.4 Hz, 3H, CH2CH3), 1.07 (doublet, J = 7.0 Hz, 3H, CHCH3), 1.37–1.45 (multiplet, 1H, CH2CH3), 1.50–1.57 (multiplet, 1H, CHCH3), 1.92–2.08 (A,B) (multiplet, 1H, CHCH3), 3.26 (doublet of doublets, J1 = 14.9 Hz, J2 = 4.4 Hz, 1H, PCH).
B: 31P NMR (D2O, δ, ppm): 12.79 1H NMR (D2O, δ, ppm): 0.94 (triplet, J = 7.4 Hz, 3H, CH2CH3), 1.11 (doublet, J = 7.0 Hz, 3H, CHCH3), 1.21–1.29 (multiplet, 1H, CH2CH3), 1.67–1.74 (multiplet, 1H, CHCH3), 1.92–2.08 (A,B) (multiplet, 1H, CHCH3), 3.19 (doublet of doublets, J1 = 14.2 Hz, J2 = 6.2 Hz, 1H, PCH).
Synthesis of α-Hydroxyphosphonates
1-Hydroxy-2-methylpropanephosphonic acid (compound 4) and 1-hydroxy-2-methylbutanephosphonic acid (compound 5) were synthesized in two-step reactions. At the beginning, the diethyl 1-hydroxyphosphonates were synthesized (17). During next step, acidic hydrolysis of the obtained ester was accomplished within 6 h. The resulting solution was evaporated. The purity of the white solid was established by NMR spectroscopy.
1-Hydroxy-2-methylpropanephosphonic Acid (Compound 4)
31P NMR (D2O, δ, ppm): 24.04 1H NMR (D2O, δ, ppm): 0.91 (triplet, J = 8 Hz, 6H, CH3CHCH3), 1.90–1.95 (multiplet, 1H, CH3CHCH3), 3.55 (triplet, J =13.9 Hz, 1H, PCH).
1-Hydroxy-2-methylbutanephosphonic Acid (Compound 5)
Compound 5 was obtained as a mixture of diastereomers (A:B) in a molar ratio of 1:0.75.
A: 31P NMR (D2O, δ, ppm): 24.20 1H NMR (D2O, δ, ppm): 0.90 (triplet, J = 6.8 Hz, 3H, CH2CH3), 1.00 (doublet, J = 7.0 Hz, 3H, CHCH3), 1.28–1.36 (multiplet, 1H, CH2CH3), 1.45–1.53 (multiplet, 1H, CHCH3), 1.75–1.85 (multiplet, 1H, CH2CH3), 3.86 (doublet of doublets, J1 = 9.8 Hz, J2 = 3.5 Hz, 1H, PCH).
B: 31P NMR (D2O, δ, ppm): 24.01 1H NMR (D2O, δ, ppm): 0.89 (triplet, J = 7.0 Hz, 3H, CH2CH3), 1.02 (doublet, J = 7.0 Hz, 3H, CHCH3), 1.21–1.28 (multiplet, 1H, CH2CH3), 1.64–1.72 (multiplet, 1H, CHCH3), 1.75–1.85 (multiplet, 1H, CH2CH3), 3.69 (doublet of doublets, J1 = 7.3 Hz, J2 = 7.3 Hz, 1H, PCH).
Synthesis of N-Derivatives of α-Aminophosphonates
Compounds 6–8 were synthesized using a two-step procedure. In the first step 1-aminophosphonic acids (1-aminoethanephosphonic acid, 1-amino-2-methylpropanephosphonic acid, and 1-amino-1-phenylmethanephosphonic acid) were obtained according to the method described in the literature (16). In the second step, acetylation of the amine group using acetic anhydride was used (18). The purity of the compounds obtained were determined by NMR spectroscopy.
N-Acetyl-1-aminoethanephosphonic Acid (Compound 6)
31P NMR (D2O, δ, ppm): 22.59 1H NMR (D2O, δ, ppm): 1.23 (doublet of doublets, J = 16.4, 7.4 Hz, 3H, CH3CH), 1.92 (singlet, 3H, CH3CHO), 4.10 (multiplet, 1H, PCH).
N-Acetyl-1-amino-2-methylpropanephosphonic Acid (Compound 7)
31P NMR (D2O, δ, ppm): 21.91 1H NMR (D2O, δ, ppm): 0.87 (triplet, J = 7.4 Hz, 6H, (CH3)2CH), 1.96 (singlet, 3H, CH3), 1.98–2.11 (multiplet, 1H, (CH3)2CH), 3.94 (doublet of doublets, J = 17.5, 5.5 Hz, 1H, PCH).
N-Acetyl-1-amino-1-phenylmethanephosphonic Acid (Compound 8)
31P NMR (D2O, δ, ppm): 17.48 1H NMR (D2O, δ, ppm): 1.96 (singlet, 3H, CH3), 5.12 (doublet, J = 20.9 Hz, 1H, PCH), 7.19–7.46 (multiplet, 5H, Ph).
Preparation of NMR Samples
α-Cyclodextrin (100 mM) was dissolved in D2O (600 μL). Next, 10 mM of a particular phosphonate compound was added and the pH value of the solution was adjusted to the proper pD with NaOD or DCl solutions.
Results and Discussion
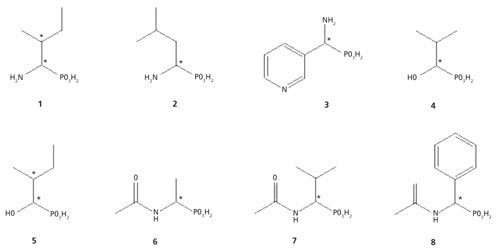
The goal of this work was to define a quantitative protocol that would allow the individual enantiomers of chiral organophosphorus compounds 1–8 to be determined by 31P NMR spectroscopy. The simplicity and speed of the determination using the protocols described here are an unquestionable advantage compared to other methods. This is a useful alternative, especially for process monitoring, which would require the assessment of enantiomeric purity of samples at low volumes and product concentrations in real time. Methods were developed for structurally diverse compounds with both aliphatic and aromatic side chains. To achieve the desired spectroscopic resolution of enantiomers, solutions of α-cyclodextrin and organophosphorus compounds were mixed together. It is noteworthy that smaller size molecules fit better into the α-CD cavity. For every chiral substrate, it was necessary to perform preliminary individual experiments, for establishing the optimum conditions for NMR analysis. Thus, the samples were prepared in acidic, neutral, and basic solutions (for example, compound 3, Figure 2). Measurements under acidic and basic conditions reflect the two different ionized states of phosphonic acid, namely the mono and doubly ionized state. The pD of the particular solution strongly influences the degree of enantiomeric resolution because it influences the ability to form inclusion (guest–host) complexes with the chiral solvating agent, which is constructed with the functionalities of defined polarity. In the case of a compound with two stereogenic centers, the use of α-cyclodextrin allowed 31P NMR spectroscopy for the detection and quantification of the enantiomeric excess of diastereomeric complexes. Thus, the best result for resolution of the 1-amino-2-methylbutanephosphonic acid (compound 1) diastereomeric isomers was obtained at pH 7 (100 mM α-CD) (Figure 3), where all four isomers are separated quite well. Good water solubility of α-CD allows the use of the ratio substrate: chiral solvating agent (1:10, C–C). Such a sample composition allowed effective formation of the α-CD complexes and enabled rapid spectral acquisition and reliable integration of 31P NMR resonances. The interactions between the chiral solvating agent and the compounds being studied are based on the presence of hydrogen bonds, dipole-dipole interactions, and steric effects that occur inside of the cavity of α-CD (4,12–14).
Figure 2: 31P NMR spectra of 10 mM 1-amino-2-methylpyridine phosphonic acid (compound 3) with α-CD (100 mM): 1, pD ≈ 2; 2, pD ≈ 7; and 3, pD ≈ 10.
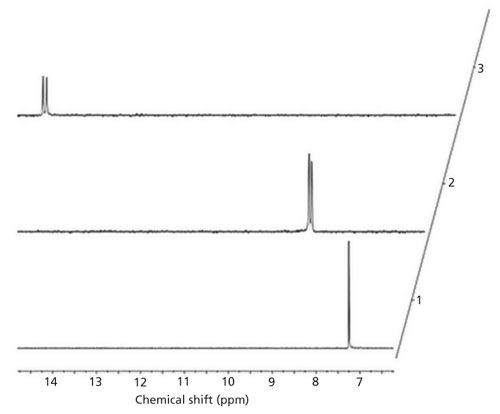
Figure 3: Figure 3: 31P NMR spectrum of complex 1-amino-2-methylbutanephosphonic acid (compound 1) and α-CD at pD = 7.08.
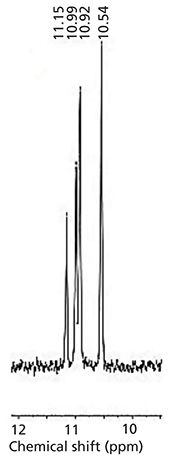
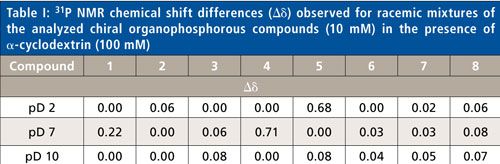
The 31P NMR chemical shifts recorded for chiral compound mixtures composed of two (compounds 2–4 and compounds 6–8) or four enantiomers (compounds 1 and 5) in the presence of α-CD are summarized in Table I. For the aminophosphonic analog of leucine (compound 2), the best results were obtained in acidic solutions. The complexed aminophosphonic acid with α-CD is more stable under these conditions, possibly because the protonated form is preferred. For hydroxyphosphonate (compound 5), acidic conditions were required. This would suggest that the mono-ionized form of phosphonic group (PO3-) is better for complexation with the α-CD (4). However, clear signal separation in compounds 1 and 4 were obtained at the values of pD = 7. For the aminophosphonic acid containing two stereogenic centers (that is, compound 1), the inclusion complex with chiral solvating agent was stable and four isomers were observed in the 31P NMR spectrum. At neutral pD, compound 1 is in the zwitterionic form, which probably facilitates complexation and enantiomeric excess evaluation. Moreover, for compound 4, the best enantiodiscrimination is achieved under neutral (pD = 7) conditions. This could be attributed to a change in the ionization state of phosphonic group from PO3H- to PO32. It also means, that at pD ≈ 7 is close to the pKa of the less acidic hydroxyl moiety of phosphonic group (4). A basic solution was essential for the analysis of 1-amino-2-methylpyridine phosphonic acid (compound 3) (Figure 2). Under such conditions, the phosphonic acid moiety of guest compound is deprotonated and hydroxyl groups inside the α-CD cavity are protonated, which stabilizes the guest-host complex. An alkaline environment improves the solubility of aminophosphonates, which is crucial for the NMR analysis. Results obtained for compounds 6–8 are in an excellent agreement with the previous work of Kozyra and colleagues (10). Determination of enantiomeric excess for N-acetyl-1-aminoethanephosphonic acid (compound 6), N-acetyl-1-amino-2-methylpropanephosphonic acid (compound 7), and N-acetyl-1-amino-1-phenylmethanephosphonic acid (compound 8) (Table I) were pH-independent. This suggests that the N-blocked aminophosphonic acids over the whole pD range exist in the same ionization state. These results also suggest that the amino-functionality ionization is a crucial factor for the complexation process. The better separation of signals (dispersion) in the NMR spectrum for these compounds under basic condition was a result of improved solubility of the samples and a slightly better fit between the guest and host in the cavity of the chiral solvating agent.
Conclusions
The results described in this article illustrate that resolution of phosphonate derivatives into their respective enantiomers can be determined by quantitative 31P NMR spectroscopy, but the dispersion of signals is strongly dependent on the pD of the substrate solutions containing α-cyclodextrin. The pD values also strongly influence substrate solubility, the ability of the guest-host complex of α-CD–organophosphonic acid to form, and the quality (signal dispersion) of the enantiodiscrimination using 31P NMR spectroscopy. Optimization of sample pDs are an essential feature for successful enantiomeric excess analysis. The principal advantages of the approach presented here are cost, simplicity of the method, and the speed of the measurement. The technique can also be applied to biocatalytic processes and for the direct monitoring of the enantiodiscrimination progress (during bioconversion).
Acknowledgment
This work was financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of WrocÅaw University of Science and Technology.
References
- B. Nowack, Water Research37, 2533–2546 (2003).
- N. Kmiecik, M. Klimek-Ochab, M. Brzezinska-Rodak, P. Majewska, and E. Zymanczyk-Duda, Biotechnol. Res. Int.http://dx.doi.org/10.1155/2013/927361 (2013).
- E.D. Naydenova, P.T. Todorov, and K.D. Troev, Amino Acids38, 23–30 (2010).
- E. Rudzinska, G. DziedzioÅa, Å. Berlicki, and P. Kafarski, Chirality22, 63–68 (2010).
- O.I. Kolodiazhnyi, Tetrahedron- Asymmetr. 16, 3295–2240 (2005).
- M. Brzezinska-Rodak, M. Klimek-Ochab, E. Zymanczyk-Duda, and P. Kafarski, Molecules16, 5896–5904 (2011).
- Z.H. Kudzin, D.K. Gralak, G. Andrijewski, J. Drabowicz, and J. Åuczak, J. Chromatogr. A998, 183–199 (2003).
- G.S. Prasad, J.R. Krishna, M. Monajunath, O.V.S. Reddy, M. Krishnaiah, C.S. Reddy, and V.G. Puranik, Arkivoc13, 133–141 (2007).
- E. Rudzinska, P. Dzygiel, P. Wieczorek, and P. Kafarski, J. Chromatogr. A979, 115–122 (2002).
- K. Kozyra, M. Klimek-Ochab, M. Brzezinska-Rodak, and E. Zymanczyk-Duda, Cent. Eur. J. Chem. 11, 1542–1547, (2013).
- S. Wren, Chromatographia Suppl. 54, 59–77 (2001).
- Å. Berlicki, E. Rudzinska, and P. Kafarski, Tetrahedron-Asymmetr. 14, 1535–1539 (2003).
- Å. Berlicki, E. Rudzinska, A. Mucha, and P. Kafarski, Tetrahedron-Asymmetr. 15, 1597–1602 (2004).
- E. Rudzinska, Å. Berlicki, A. Mucha, and P. Kafarski, Tetrahedron-Asymmetr. 18, 1579–1584 (2007).
- B. Boduszek, Tetrahedron52, 12483–12494 (1996).
- J. Oleksyszyn and R. Tyka, Tetrahedron Lett.32, 2823–2824, (1977).
- P. Majewska, M. Doskocz, B. Lejczak, and P. Kafarski, Tetrahedron-Asymmetr. 20, 1568–1574 (2009).
- U. Niewohner, D. Schauss, M. Hendrix, G. Konig, F.G. Boss, F.J. Van Der Staay, R. Schreiber, K.H. Schlemmer, and R. Grosser, Substituted imidazotriazinones. US 2002/0198377 A1 (2002).
Natalia Kmiecik, Monika Serafin, and Ewa Zymanczyk-Duda are with the Department of Bio-organic Chemistry, Faculty of Chemistry at WrocÅaw University of Science and Technology in WrocÅaw, Poland. Tomasz K. Olszewski is with the Department of Organic Chemistry, Faculty of Chemistry at WrocÅaw University of Science and Technology. Direct correspondence to: natalia.kmiecik@pwr.edu.pl

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.