Study of the Content of Inorganic Arsenic and Heavy Metals in Samples of Rice Cakes in the City of Madrid
Spectroscopy
Study of the Content of Inorganic Arsenic and Heavy Metals in Samples of Rice Cakes in the City of Madrid
This study analyzes the presence of inorganic arsenic and heavy metals in different varieties of rice cakes, both so-called "bio" (organic) and "non-bio," as well as those made with white rice and brown rice. Determination of cadmium (Cd), mercury (Hg), nickel (Ni), and lead (Pb) has been carried out by inductively coupled plasma mass spectrometry (ICP–MS) for samples previously digested in a microwave oven. The analysis of inorganic arsenic has been carried out by liquid chromatography (HPLC) combined with inductively coupled plasma–mass spectrometry (HPLC-ICP–MS) prior to acid extraction. All samples analyzed are below the limits established by local regulations. However, the content found for inorganic arsenic, cadmium, and nickel may be a risk to health for children, who are the marketing target for some of the rice cake products.
Today, societal concerns about maintaining a healthy and balanced diet is causing an increase in the consumption of certain foodstuffs that are apparently healthier. It should be noted there is a growing interest among consumers about rice cakes, eaten as an appetizer or a snack replacement for more caloric foods, such as sweet cakes or snack chips. It is possible, however, that these rice products are not as healthy as they seem, because there are studies that demonstrate the presence of certain contaminants. In spite of this, a variety of rice cakes are marketed for consumption by the entire population, and in some cases intended specifically for infants and children.
As a result of this trend toward consumption of healthy products, there is also a boom in the production and consumption of products categorized as organic, or bio, as opposed to normal, or non-bio products. For consumers, organic products are more popular, because they are considered to be of higher quality and believed to be free of possible contaminants that normal products may have.
Numerous studies (1–5) have been published showing disturbing amounts of inorganic arsenic (iAs) in these products. In this regard, the national food agency of Sweden (National Food Agency, NFA) conducted a study (6) on the content of inorganic arsenic in rice products in 2015 and recommended that children under six years old should not be fed rice cakes, due to their high content of iAs.
Arsenic is a metal that is found naturally in the Earth's crust; it is present in the air, water, and soil. In the case of food, it can occur in several different chemical forms, both organic and inorganic. Nowadays, there is scientific evidence demonstrating that inorganic arsenic, both As (III) and As (V), is the most toxic and harmful to health, because it has been proven that it can cause problems related to fetal development, neurotoxicity, diabetes, and cardiovascular diseases. In addition, the International Agency for Research on Cancer has classified it as "carcinogenic to humans" (7), because it can cause skin cancer, bladder cancer, and lung cancer.
The scientific technical committee on contaminants in the food chain (Contam) of the European food safety authority (EFSA) adopted an opinion (8) on arsenic in food. It modifies the provisional tolerable weekly intake (ISTP), established by the Joint Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) (FAO/WHO) Expert Committee on Food Additives (JECFA), based on the existence of studies demonstrating the toxicity of the iAs with exposure levels lower than those reviewed by JECFA. Thus, Contam set a limit of confidence between 0.3 and 8 µg/kg of body weight per day.
Scientific opinion (8) established that children under three years of age were those most exposed to foods with iAs, because rice is a predominant ingredient in a wide range of foods for this group. In general, it is estimated that dietary exposure to iAs of infants and young children is between double and triple that of adults.
In this context, this study has been carried out for the determination of iAs and expanded to included the analysis of various heavy metals, in order to conduct a more exhaustive study of this type of food. The specific heavy metals analyzed were cadmium, mercury, nickel, and lead.
The concentration of these heavy metals can be considered especially relevant when dealing with toxic elements in doses that are harmful to the health of consumers. Except for Ni, these heavy metals are regulated, and in numerous scientific studies, their presence has been demonstrated in food and the consequences of their consumption have been studied.
The European Union (EU) has approved Commission Regulation (EU) 2015/1006 (9), which modifies the Commission Regulation (CE) 1881/2006, in which the maximum content of iAs for rice and rice products is established. In a similar way, measures at the global level (CODEX Alimentarius) have also been adopted, so it is possible to carry out control measures of iAs in rice and products derived from rice.
Commission Regulation (EU) 488/2014 (10), Commission Regulation (EU) 2015/1005 (11), and Commission Regulation (EU) 2018/73 (12) establish maximum limits for Cd, Pb, and Hg in foods, respectively. However, there are no limits in the case of Ni. Currently, European authorities are conducting a study (13) to establish maximum limits for this element in foodstuffs, as indicated in the Commission Recommendation (EU) 2016/1111 of 6 July 2016 on the monitoring of nickel in food (14).
The samples analyzed in this study correspond to different varieties of rice cakes, both with the "bio" (organic) designation and without it, and rice cakes made with white and brown rice, all sold in the city of Madrid. In total, 29 samples of rice cakes were analyzed, of which 11 were labelled as "bio," while the remaining 18 did not have this designation. Note that 10 of the analyzed samples were made with white rice, and 19 with brown rice, because different studies have shown different amounts of iAs in white rice and brown rice.
Experimental
Instrumentation and Operating Conditions
The samples were digested in a microwave oven (Milestone model Ethos One) equipped with output power regulation and automatic control systems for temperature and pressure of all the vessels, which allows for the monitoring of both parameters during the process. The containers used for samples are PTFE tubes with lids, with a capacity of 100 mL; they were previously subjected to a rigorous cleaning process, according to the manufacturer's instructions.
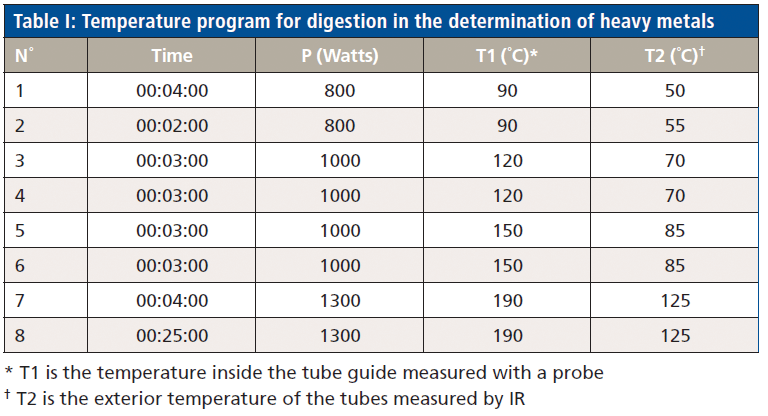
The temperature program used for digestion is detailed in Table I (for the determination of heavy metals) and Table II (for the determination of iAs).
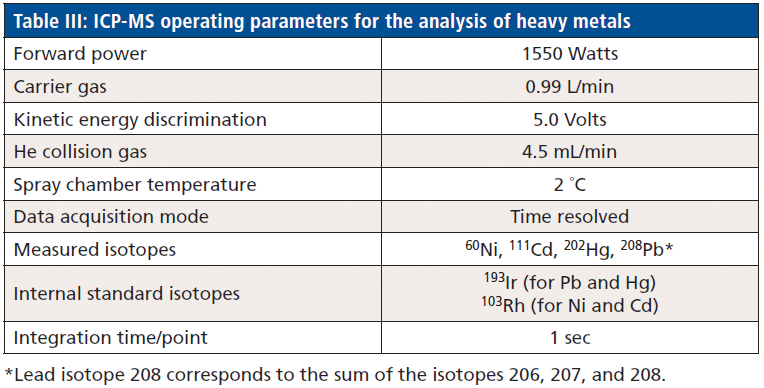
For the analysis of heavy metals, an inductively coupled plasma–mass spectrometry (ICP–MS) instrument (Agilent Technologies model 7900), equipped with an 89 position integrated autosampler (IAS), was used. The operating parameters of the ICP–MS instrument are outlined in Table III.
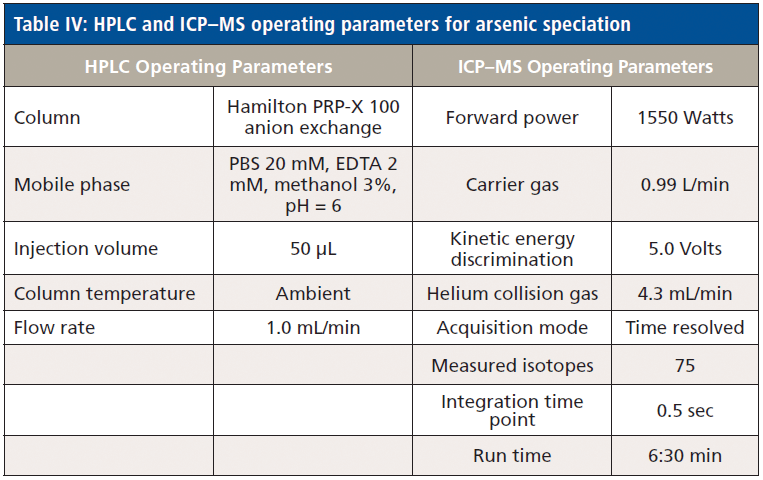
Inorganic arsenic (iAs) determinations were performed using a high performance liquid chromatography (HPLC) instrument (Agilent Technologies model 1200 Infinity), equipped with a model 1260 Quat Pump VL quaternary pump, an injector (model 1260 ALS), and a column compartment (model 1260 TCC). This HPLC system was used in conjunction with the ICP–MS instrument referred to above. The operating parameters of this system are listed in Table IV.
The volumetric materials used for all solutions were calibrated polypropylene single-use tubes (DigiTubes SCP Science), except for the analysis of iAs, for which centrifuge tubes, also single-use, were used (VWR Metal Free, 15 mL volume). Eppendorf brand micropipettes were used for sampling corresponding aliquots.
Reagents and Standards
For the preparation of the reagents and solutions for washing and rinsing of the work material, deionized water (H2O) class type I (resistivity: 18.2 MO cm) was used, purified using Milli-Q Integral system. The nitric acid (HNO3, 65%) and hydrochloric acid (HCl, 37%) utilized are quality pro analysis (p.a.), supplied by Scharlau, and distilled in the laboratory by a Savillex distiller model DST-1000. The hydrogen peroxide (H2O2) was from Merck, 30%, Suprapur. Glacial acetic acid was from Scharlau, 99.8%, ultratrace and ppb-trace analysis grade. The tuning solution for the ICP–MS instrument (Agilent Technologies) contained 1 µg/L Ce, Co, Li, Mg, Tl and Y. Finally, the argon and helium (99.999%) used in plasma generation and interference elimination were supplied by CONTSE.
In the case of the determination of heavy metals, all standard solutions were prepared with a mixture of 2% HNO3 and 1% HCl, based on the following standards, having concentrations of 1000 mg/L: Cd (Sigma-Aldrich), and Hg, Ni and Pb (VHG-Labs).
Intermediate solutions of elements of interest were prepared as follows: Cd (1 mg/L), Hg (1 mg/L), Ni (10 mg/L), and Pb (1 mg/L) were used to prepare a multielemental standard (composed of 4 µg/L Cd, 5 µg/L Hg, 50 µg/L Ni, and 10 µg/L Pb) which is later used to prepare the calibration standards. To do this, the standard is taken and diluted 100 times, 25 times, 10 times, and 2.5 times, to obtain a calibration function with 5 levels.
For validation of Cd, Ni, and Pb, a multi-element standard of 1 µg/L was prepared from a commercial standard of 100 mg/L, provided by Scharlau, and a standard of 1.016 µg/L Hg was prepared from a 0.1016 mg/L NIST reference material, used for verification of the Hg control. Finally, 400 µg/L of Ir and Rh, prepared in a solution with 2% HNO3, 1% HCl, and 5% acetic acid, was used as the internal standard solution. This internal standard was prepared from solutions of 1000 mg/L of Ir (Sigma-Aldrich) and Rh (Panreac).
For the analysis of inorganic arsenic, the solution used for extraction is 0.2% HNO3 and 1% H2O2, noting that the solution used for the preparation of all standards was 0.2% HNO3.
For inorganic As, the calibration standards were prepared from a standard of As (V) (from As2O5) of 100 mg/L in H2O (VHG Labs). Using an intermediate solution of 1 mg/L standard with 10 µg/L prepared, this ssolution was diluted 2.5 times, 10 times, 25 times and 50 times, to obtain a calibration function with 5 levels.
An analysis of another As (V) reference material of was included, with the same specifications as the previous one, but in a different batch. Furthermore, a control of the process of oxidation of As (III) to As (V), using a standard of As (III), supplied by VHG Labs (from As2O3); 100 µg/mL in 2% HCl, was included.
The mobile phase composition was 20 mM PBS / 2 mM EDTA / 3% MeOH, in deionized water class type I, pH = 6, using Panreac methanol (CH3OH) with a minimum assay of 99.9%, pure Panreac disodium ethylenediaminetetraacetate dihydrate (EDTA, with an assay of 99.0–101.0%, and pure Panreac sodium dihydrogen phosphate dihydrated (assay 98.0–100.5%).
Sample Treatment and Extraction Method
The steps of the analytical process are shown in Figure 1.
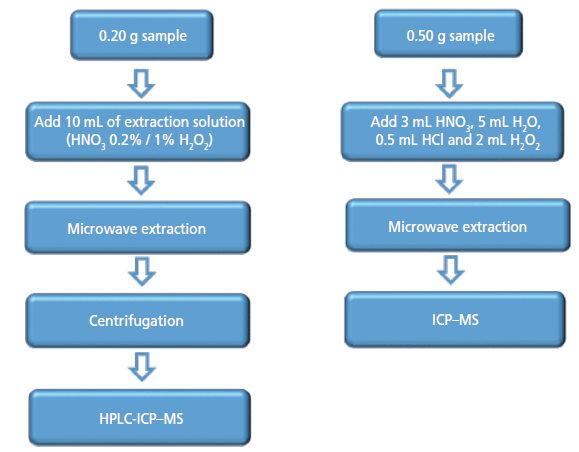
Figure 1: The steps in the analytical method used in this study.
The homogenization of all samples was achieved by following the method described in the standard UNE-EN 13804:2013 (15), using a grinder to obtain a representative sample of the product to be tested.
Determination of Heavy Metals
To begin, the homogenized samples were weighed to 0.50 ±0.05 g. For the digestion process, 3 mL HNO3, 5 mL H2O, 0.5 mL HCl, and 2 mL of H2O2 were added and digested in the microwave (conditions described in Table I), and, once the process was complete, samples were left to cool down to room temperature. The solutions were then transferred to the DigiTubes filled with up to 50 mL deionized water. These prepared samples were then ready to be analyzed by ICP–MS.
Determination of Inorganic Arsenic
In this study, the determination of inorganic arsenic was carried out as the sum of arsenite (As [III]) and arsenate (As [V]). This quantifies the peak corresponding to the As (V) obtained with a retention time of 4.75 min (see Figure 2).
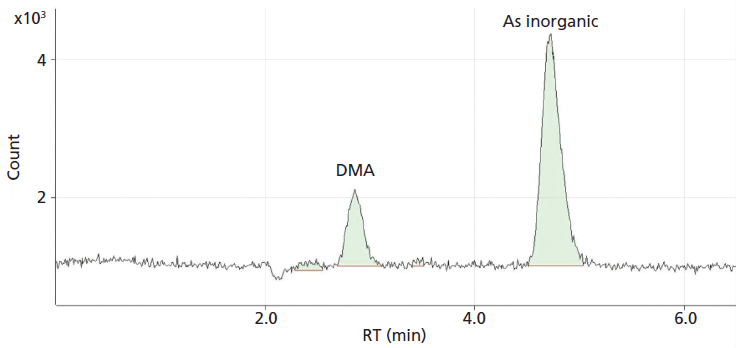
Figure 2: Arsenic speciation chromatogram showing a peak at 4.75 min for arsenate (As V).
The homogenized sample has been weighed 0.2 ±0.02 g, and 10 mL of the solution prepared for extraction has been added. Later, the sample was treated in the microwave (conditions described in Table II). In this process, all of the As (III) in the sample is oxidized to As (V). Once the process has been completed, samples are left to cool down to ambient temperature, transferred to 15 mL tubes, and centrifuged for 10 min at ≥4000 rotations per min (rpm) The supernatant is filtered using 0.45 µm pore size nylon filters, and is poured into 1 mL polypropylene vials to inject into the HPLC instrument.
Validation and Quality Control
The analytical method used for the determination of heavy metals is required to meet the criteria of the Commission Regulation (EC) N° 333/2007 of March 28, 2007 (16) laying down the methods for sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-monochloropropanediol (3-MCPD), and benzo(a)pyrene in foodstuffs and its modifications. Validation has been carried out according to the requirements of the standard UNE/EN-ISO 17025 (17) for testing laboratories, and it is accredited by the Entity National Accreditation (ENAC). In the case of the determination of iAs, the analytical method is in process of accreditation. The results of the validation are shown in Table VI.
For quality control, we have participated in different intercomparison exercises and additions of known concentrations of the analyte used to assess the accuracy of the method. In the case of iAs, a standard of As (III) has been used as described in Section 2.2.
Results and Discussion
The results obtained in this study are shown in Table V, detailing the maximum concentrations and average concentrations obtained for iAs, Cd, Ni, and Pb, as well as the number of samples that have been quantified in each case. The results for Hg are not included in this table, because the concentrations obtained are <0.010 mg/kg, and they have not, therefore, been able to be accurately quantified.
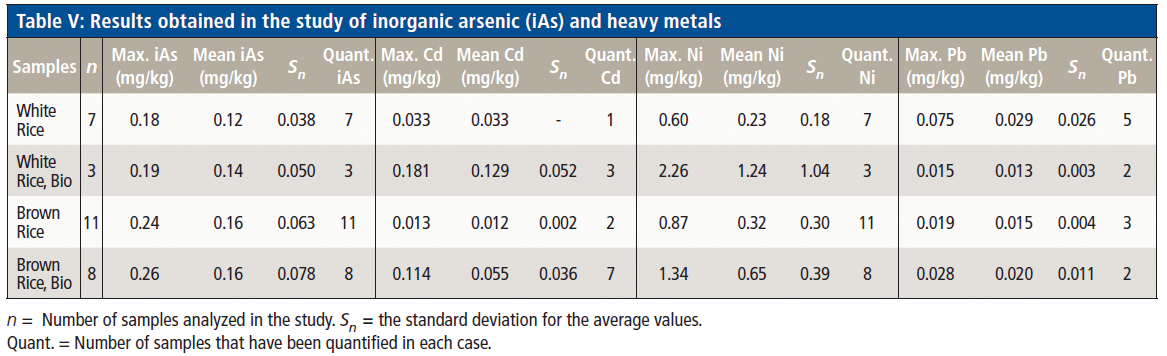
All the results obtained in the determination of iAs were able to be quantified. Nevertheless, it should be noted that no sample exceeded the maximum value established by Commission Regulation (EU) 2015/1006 (9) regarding maximum levels of inorganic arsenic in foodstuffs, set at 0.30 mg/kg for rice cakes. However, taking into account a value of 0.3 µg/kg of body weight as the threshold of tolerable risk considered in the studies carried out by the EFSA (8), in the case of a six-year-old child (with an approximate weight of 20 kg), for example, the admissible threshold would be 6 µg of iAs, and the consumption of a single rice cake (about 30 g), with the highest value of found iAs (0.256 mg/kg) would result in the intake of approximately 7.7 µg of iAs. Therefore, special attention should be given to rice cake consumption by children.
In the case of Pb, the results obtained are not considered significant, because the concentrations were below the thresholds referred to in toxicity studies published for this heavy metal (18).
In terms of the results obtained for Cd, 60% of the analyzed samples are quantified as having a concentration >0.010 mg/kg. It should be noted that all of these results are below the reference value in the Commission Regulation (EU) 488/2014 (10), where the maximum content of Cd in rice has been established as 0.20 mg/kg. However, health authorities establishes a tolerable daily intake of 0.36 µg/kg body weight (19) of this heavy metal to ensure all consumers have sufficient protection. Referring to a previous example, a six-year-old child should not exceed 7.1 µg of Cd daily intake. Hence, based on the data obtained in this study and where the maximum found value was present (0.181 mg/kg), consumption of a single rice cake would contribute 5.4 µg of Cd.
On the other hand, the values obtained for Ni show a high concentration of this metal in rice cakes, levels that even exceeded the concentrations of other contaminants that are regulated. Taking as a reference the measured maximum value, the consumption of a single rice cake would contribute up to 67.8 µg of Ni, which, at first sight, could be considered relevant if compared to the established maximum levels for other metals.
In addition, in the case of iAs, there are no significant differences between the rice cakes considered to be "bio" and "non bio"; on the other hand, more iAs is seen in the rice cakes made with brown rice than those made with white rice. The content of iAs is not affected by the type of rice cultivation (bio or non bio), but the processing of the rice has important influence as a factor that reduces the concentration of iAs in white rice compared to brown rice, as shown in Figure 3.
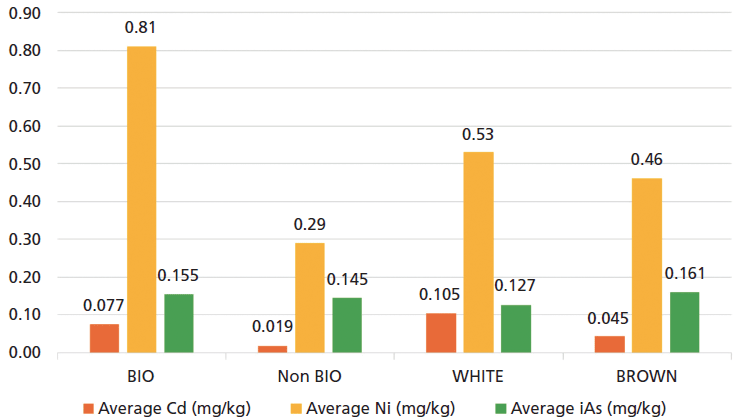
Figure 3: Results obtained in the study for cadmium, nickel, and inorganic arsenic (iAs). BIO: Total of analysed samples of rice cakes "BIO" (Brown + White); Non BIO: Total of analysed samples of rice cakes "Non BIO" (Brown + White); WHITE: Total of analysed samples of rice cakes "White rice" (BIO + Non BIO); BROWN: Total of analysed samples of rice cakes "Brown rice" (BIO + Non BIO).
The results for Cd and Ni highlight that the "bio" rice cakes present concentrations clearly higher than the "non-bio" rice cakes, as shown in Figure 3. In the case of Cd, there are also significant differences between rice cakes made with white rice versus those made with brown rice. On the contrary, in the case of Ni, we can see that there are no significant differences between the content of these metals in either variety of cake.
In this case, based on the determined quantities of Cd and Ni, we can appreciate a large influence in the type of rice cultivation but not so much in the processing of the rice.
Conclusions
All rice cakes samples analyzed comply with the legislation in force, because they do not exceed the maximum levels permitted; therefore, these foods should be considered safe for consumption.
However, based on the results obtained in this study for iAs, Cd, and Ni, it would be advisable that consumption of rice cakes by children be regulated by health authorities and that greater control be maintained over rice cakes that have with both the designation of "bio" and are made from brown rice.
On the other hand, the test method used for the analysis of Cd, Hg, Ni, and Pb by microwave acid digestion and determination by inductively coupled plasma–mass spectrometry is considered a valid option to carry out a study as presented in this publication, obtaining results in accordance with the requirements of the UNE-EN ISO 17025 standard.
The coupling of liquid chromatography to ICP–MS is a recommended method for chromatographic separation of the different species of arsenic, for the analysis of inorganic arsenic, determined as the sum of As (III) and As (V).
References
(1) R. Guillod-Magnin, B.J. Brüschweiler, R. Aubert and M. Haldimann, Arsenic species in rice and rice-based products consumed by toddlers in Switzerland. Food Addit.Contam. Part A 35(6), 1164-1178 (2018). DOI: 10.1080/19440049.2018.1440641.
(2) A.J. Signes-Pastor, M. Carey, and A.A. Meharg, Food Chem. 191, 128–134 (2016).
(3) M. Molin, S.M. Ulven, H. Margrete Meltzer, and J. Alexander, J. Trace Elem. Med. Biol. 31, 249–259 (2015).
(4) H.N. Lynch, G.I. Greenberg, M.C. Pollock, and A.S. Lewis, Sci. Total Environ. 496, 299–313 (2014).
(5) N. Ferré-Huguet, R. Martí-Cid, M. Schuhmacher, and J.L. Domingo, Biol. Trace Elem. Res. 123(1–3), 66–79 (2008).
(6) Livsmedelsverket National Food Agency, Sweden. 2015. Study reveals problems with arsenic in rice and rice products. https://www.livsmedelsverket.se/en/about-us/press/study-reveals-problems-with-arsenic-in-rice-and-rice-products.
(7) International Agency for Research on Cancer (IARC). Monographs on the Identification of Carcinogenic Hazards to Humans. List of classifications, volumes 1-123. https://monographs.iarc.fr/list-of-classifications-volumes/
(8) EFSA (European Food Safety Authority): EFSA Panel on Contaminants in the Food Chain (CONTAM): Scientific Opinion on Arsenic in Food. EFSA Journal 7(10), 1351 (2009).
(9) Commission Regulation (EU) 2015/1006 of 25 June 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels of inorganic arsenic in foodstuffs.
(10) Commission Regulation (EU) No 488/2014 of 12 May 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs.
(11) Commission Regulation (EU) 2015/1005 of 25 June 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs.
(12) Commission Regulation (EU) 2018/73 of 16 January 2018 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for mercury compounds in or on certain products.
(13) EFSA (European Food Safety Authority): EFSA Panel on Contaminants in the Food Chain (CONTAM): Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA Journal 13(2), 4002 (2015).
(14) Commission Recommendation (EU) 2016/1111 of 6 July 2016 on the monitoring of nickel in food.
(15) UNE-EN 13804:2013. Foodstuffs - Determination of elements and their chemical species - General considerations and specific requirements.
(16) Commission Regulation (EC) No 333/2007 of 28 March 2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo(a)pyrene in foodstuffs.
(17) General requirements for the competence of testing and calibration laboratories (ISO/IEC 17025:2017).
(18) EFSA (European Food Safety Authority): EFSA Panel on Contaminants in the Food Chain (CONTAM): Scientific Opinion on Lead in Food. EFSA Journal 8(4), 1570 (2013).
(19) EFSA (European Food Safety Authority). 2012. Scientific Report: Cadmium dietary exposure in the European population. EFSA Journal 10(1), 2551 (2012).
Javier Peinador Asensio, Silvia Borobia Caminos, and Pilar Jiménez Navarro are with the Public Health Laboratory of Madrid, Spain. Direct correspondence to: peinadoraj@madrid.es

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.
Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.