Studying Inorganic Arsenic, Heavy Metals, and Iodine in Dried Seaweed
In this study, 33 different dried seaweed products were purchased in local markets in Madrid and analyzed. Arsenic, cadmium, mercury, nickel, lead and iodine were determined by inductively coupled plasma–mass spectrometry (ICP-MS) in samples previously digested in a microwave oven. In addition, inorganic arsenic (iAs) was determined by high performance liquid chromatography (HPLC) combined with microwave-assisted extraction followed by ICP-MS. Results indicate that the levels of Cd, Ni, and Pb were relevant in some of the samples, and may pose a risk to consumers. The concentrations of Hg were overall the lowest found in the samples. Hijiki seaweed (Hizikia fusiforme) contained a considerable iAs fraction, whereas all the other seaweed species contained minimal fractions. Lastly, iodine content in most of the seaweeds surpassed the tolerable daily intakes recommended by the European Food Safety Authority (EFSA), which may pose a risk to consumers.
Seaweed has been a part of Asian diets since ancient times, but only more recently has it become a popular ingredient in Western diets because of its potential as an alternative and healthy source of food. Other applications of seaweed are also being explored, such as in food additives, feeds, fertilizers, biofuels, cosmetics, and medicines (1).
Current research on the health benefits of seaweed reveals that it contains various important vitamins (A, B1, B12, C, D, and E), minerals (potassium, sodium, phosphorus, iodine), and compounds with remarkable antioxidant capacities (2–5). Seaweed also has a low-calorie content (between 1% and 5% dry weight) (5).
However, some studies highlight that there are adverse effects to consuming large quantities of seaweed because it contains an excessive amount of heavy metals, arsenic, and iodine. As a result, there are possible risks associated with consuming too much seaweed (6).
Heavy metals not only have no beneficial function in the human body, but they are also considered toxic for humans even at low concentrations. Exposure to heavy metals usually leads to negative gastrointestinal, neurotoxic, and carcinogenic effects (7).
It is widely known that marine organisms, especially seaweed, accumulate relevant amounts of arsenic. The chemical similarities between arsenate (H2AsO4-) and phosphate (H2PO4-) is the reason why both are absorbed equally through membrane transport systems. Thus, inorganic arsenic (As3+ and As5+) is introduced in these organisms and metabolized to organic arsenic species, such as arsenobetaine, monomethylarsonic acid, dimethylarsinic acid, arsenosugars, and arsenolipids (8,9). It is reported that inorganic forms of arsenic are more dangerous and toxic than organic forms of arsenic (10). For that reason, a suitable speciation analysis must be carried out to determine the levels of inorganic arsenic in seaweed.
Iodine is a diet-essential mineral because of its importance in the thyroid gland. It serves important biological and physiological functions in the human body, especially during early growth and organ development (11). Although iodine is necessary in the diet, either an excess or a deficiency in iodine may cause disorders related to the thyroid functions. The consequences of these pathologies can be very serious in children and pregnant women (12,13).
Regulation (EU) 2017/2470 established and updated the list known as the “Novel Food Catalogue” that contains all European Union’s authorized novel foods. This list included 22 different types of edible seaweed by the end of 2020, but does not specify the maximum limits of heavy metals, iodine, and arsenic (14).
Food supplements that are seaweed-based are regulated by Commission Regulation (EC) No 629/2008, which indicates the maximum amount of cadmium that food products can contain (15). However, there is currently no specific legislation about maximum limits related to seaweed as food yet. Therefore, the Europe Union published Recommendation (EU) 2018/464 on the monitoring of different elements, such as arsenic, cadmium, lead, mercury, and iodine, in edible seaweed. Recommendation (EU) 2018/464 also mentions the importance of quantifying different species of arsenic (As3+, As5+, and total arsenic) and, if possible, other relevant arsenic species. The aim for this recommendation is to gather results from different studies, which will allow us to assess the risks associated with the consumption of seaweed, and consequently set maximum levels in the future (16).
Inductively coupled plasma–mass spectrometry (ICP-MS) is a fitting technique to quantify several elements in foodstuffs at low concentrations simultaneously. However, it is important to notice several issues in using ICP-MS when analyzing inorganic arsenic and iodine.
Total arsenic and heavy metals analysis are commonly the same; the process for both first undergoes an acid digestion followed by quantification using an elemental analysis instrument. Nevertheless, inorganic arsenic analysis has significant differences that need to be considered. A regular acid digestion is not suitable because organic arsenic species would deteriorate into As3+ and As5+. Thus, it is mandatory to utilize a previous extraction followed by a separation technique. High performance liquid chromatography (HPLC) is the most common methodology for arsenic speciation because it allows for the separation of individual arsenic species so they can be quantified accurately afterwards (17).
The literature highlights the problematic sample preparation of iodine because of memory effects and its complex redox chemistry. Equation 1 reveals that at low pH (typical digestion mixture where H+ concentration is high), iodide (I-) is oxidized to volatile molecular iodine (I2) by dissolved oxygen or other oxidants:
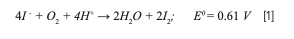
This process is favorable (Eo > 0 V), thus a regular acid digestion would lead to iodine loss and an incorrect approach to sample pretreatment, which why methods involving alkaline digestion are widely used (18,19). An alternative method being proposed in this study is performing a suitable acid digestion followed by a dilution using an alkaline solution, which is an unique and simultaneous sample pretreatment. Performing a suitable acid digestion followed by a dilution using an alkaline solution saves time and resources while taking advantage of ICP-MS multielemental analysis (20).
Materials and Methods
Instrumentation and Equipment
The cadmium, mercury, lead, nickel, total arsenic, and iodine totals were determined using an ICP-MS instrument (Agilent Technologies model 7900). An Integrated Sample Introduction System (ISIS 3) and a SPS 4 autosampler, both from Agilent Technologies, were used alongside the ICP-MS instrument, in determining the quantities of the elements mentioned above. The ICP-MS analysis operating parameters are shown in Table I.
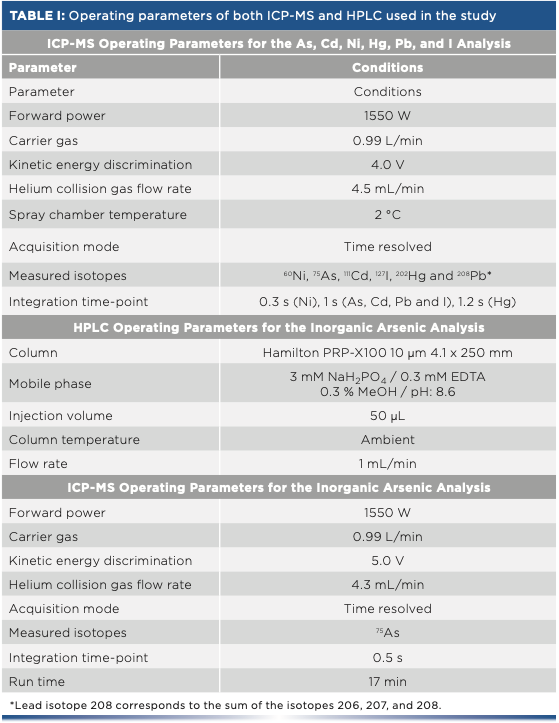
Inorganic arsenic was determined using a HPLC instrument (Agilent Technologies model 1200 Infinity) equipped with a model 1260 Quat Pump VL quaternary pump; a model 1260 ALS injector; and a model 1260 column compartment. The HPLC system was used in conjunction with the ICP-MS instrument mentioned earlier. For the inorganic arsenic determination, the operating parameters are listed in Table I.
Samples were treated in a Milestone model Ethos One microwave oven with a capacity of 10 polytetrafluoroethylene (PTFE) vessels at 100 mL each. One of the PTFE vessels is always used for a blank process.
The volumetric materials used were calibrated with polypropylene single-use tubes (DigiTubes from SCP Science), 15 mL single-use centrifuge tubes (Superclear Labcon), and a 1 L volumetric flask to prepare the mobile phases.
To centrifuge the samples, a refrigerated Lisa centrifuge from AFI groups was used.
Reagents and Standard Solutions
All solutions, cleaning procedures, digestion, and extraction methods were prepared with deionized water (H2O) class type I (according to UNE-EN-ISO 3696), and purified using a Milli-Q system from Merck. Nitric acid (HNO3 65%) pro analysis (p.a.) quality was used, supplied by Fluka and previously distilled by a Savillex distiller model DST-1000. Hydrocloric acid (HCl 37%) was ultratrace ppb-trace analysis grade from Scharlau, and hydrogen peroxide (H2O2, 30%) was suprapur grade and supplied by Merck.
Regarding the ICP-MS instrument, the tuning solution contained 10 μg/L cerium, cobalt, lithium, magnesium, thallium, and yttrium. The argon and helium used in plasma generation and interference elimination (KED mode) were both 99.999% accurate.
The alkaline solution used in the iodine determination was prepared with 40 g of NH4OH (Fluka p.a. quality), 10 g of 1-buthanol (Merck), and 1 g of ethylenediaminetetraacetic acid (EDTA) (Panreac pharma grade) mixed with 1 L of deionized water.
The extraction solution used in the inorganic arsenic determination was 0.2% HNO3 and 1% H2O2. In arsenic and heavy metals analysis, standards of 1000 mg/L arsenic, cadmium, mercury, nickel, and lead (all supplied by LGC Standards) were used. Intermediate solutions and the calibration curve were prepared in 2% HNO3 and 1% HCl (v/v). Regarding the iodine determination, a 1000 mg/L iodine standard was provided by Scharlau. Suitable intermediate solution and calibration curve were prepared in a 1:2 diluted alkaline solution. For the inorganic arsenic determination, 100 mg/L As5+ and As3+ (LGC Standards) were utilized. Moreover, monomethylarsonic acid from High Purity Standards, dimethylarsinic acid from AccuStandard, and arsenobetaine from the National Metrology Institute of Japan (NMIJ) standards, were also used to check the chromatographic separation. The intermediate solutions and the calibration curve were prepared in 0.2% HNO3 (v/v).
The internal standards used for each element in the ICP-MS analysis were germanium and rhodium from LGC Standards and iridium and tellurium from Sigma-Aldrich. A 0.4 mg/L mixture of these elements was prepared as the internal standard solution with 2% HNO3, 1% HCl, and 5% acetic acid. To stabilize the mercury signal, 0.4 mg/L of gold from SCP Science were also added.
Seaweed Samples
The seaweed samples were purchased in several markets in Madrid, Spain. To obtain representative results, numerous species of seaweed were collected.
A total of 33 samples were analyzed in this study, including two dulse (Palmaria palmata), three hijiki (Hizikia fusiforme), two kelp (Laminaria spp.), six kombu (Laminaria japonica), five nori (Porphyra tenera), one sea lettuce (Ulva lactuca), five sea spaghetti (Himanthalia elongata), and eight wakame (Undaria pinnatifida). One agar-agar (Gelidium spp.) was also included in this study because of its use in foods and cuisine. Note that the conclusions related to species with fewer than three samples should be carefully considered.
Sample Pretreatments
All seaweed were homogenized first using a coffee grinder. The different methods are detailed in Figure 1.
FIGURE 1: A summary of the determination methods used in the study.
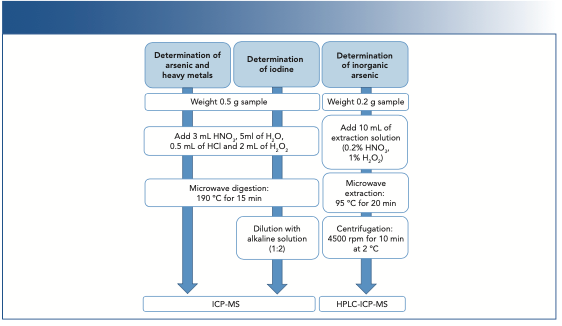
Determination of Iodine, Arsenic, and Heavy Metals
First, the homogenized samples were weighed into polytetrafluoroethylene (PTFE) vessels, and the digestion mixture of acids was added. Next, the samples were placed in the microwave oven. Once the digestion was complete and the samples cooled down to room temperature, they were transferred to DigiTubes and the tubes were filled with deionized water. An aliquot was taken and diluted with the alkaline solution (the ratio for the sample–alkaline solution was 1:2) for the parallel iodine analysis. These solutions were ready to be measured if no more dilutions were needed.
Determination of Inorganic Arsenic
Inorganic arsenic is determined as the sum of arsenate (As5+) and arsenite (As3+). The homogenized sample was weighed, mixed with the extraction solution, and introduced to the microwave oven. Validation proved that all As3+ transformed into As5+ entirely, which means that As5+ determined is equal to the complete inorganic fraction. After the microwave-assisted extraction, samples were centrifuged, diluted (if necessary), and filtered through a 0.45 μm polyvinylidene fluoride (PVDF) filters.
Validation and Quality Control (QC)
The determination methods of this work meet the criteria established in Commission Regulation (EC) No 333/2007 of March 28, 2007.
Moreover, the laboratory is accredited according to ISO/IEC-17025 requirements. The studied heavy metals, total, and inorganic arsenic in foodstuffs are included in the schedule of accreditation.
Regarding the iodine determination, the analysis was not included in the schedule of accreditation then. Therefore, the laboratory participated in a proficiency test (FAPAS-Food Chemistry Proficiency Test 07380) to evaluate method accuracy and obtained a satisfactory result (z-score = 0.3).
Results and Discussion
This work aims to provide information about the potential risks involved in seaweed consumption. For that purpose, the percentage contribution (%) by a daily portion of seaweed to a reference daily intake is estimated.
First, it is necessary to describe the typical portion of seaweed consumed in a day. Normally, labels on seaweed products give some instructions about how to cook them (an amount between 2 and 5 g is usually recommended). The literature also mentions a general daily intake of seaweed depending on the region. For example, the literature recommends estimating daily intake at 8 g/day in Asian cultures (4), 4–7 g/day in Japan (21), and <5.2 g/day in Western diets (22).
Second, a reference intake based on health impact is needed. European Food Security Authority (EFSA) scientific reports establish recommended intake values of the studied elements in foods considering their potential health risks. These values are often expressed in μg/kg bodyweight (b.w.) per day or per week, which means an average human bodyweight must be established to make the assessment.
Finally, once the results are obtained, the daily intake contribution for each element can be estimated using equation 2:
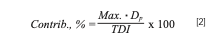
where Max. is the highest result found of the corresponding element in seaweed (mg/kg), and the maximum value obtained allow to assess the worst-case scenario when discussing the results; Dp is the daily portion consumed of seaweed (kg/day); and TDI is the tolerable daily intake according to scientific reports published by EFSA (in mg/day). To estimate this contribution, a reasonable daily portion of 5 g and an average 60 kg (~132 lbs.) body weight are set.
The overall results are summarized in Table II. Concentrations, averages, and ranges obtained are shown as well as the number of samples quantified. The percentage of inorganic arsenic related to total arsenic is shown as well, and it is also displayed in the reference intakes from EFSA and its corresponding contributions (23–27). Moreover, individual results of each sample are shown in Figure 2.
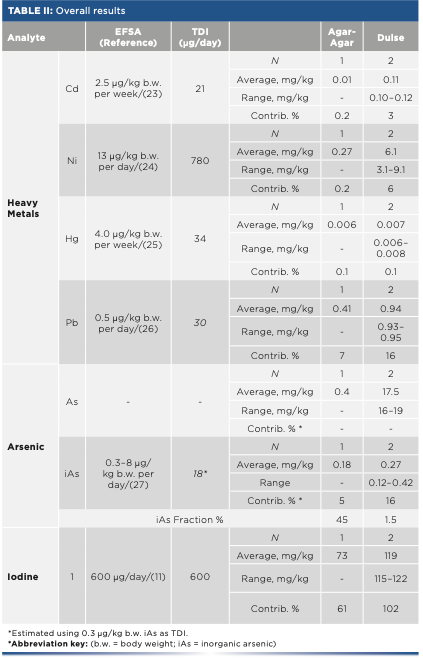
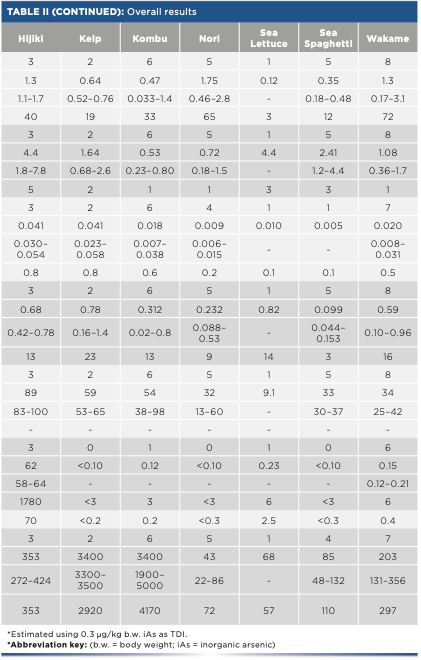
FIGURE 2: Individual results of arsenic (As), iodine (I), and heavy metals, such as cadmium (Cd), lead (Pb), mercury (Hg), and nickel (Ni) in each seaweed species. X-axis is the seaweed species and y-axis is the concentration results in mg/kg.
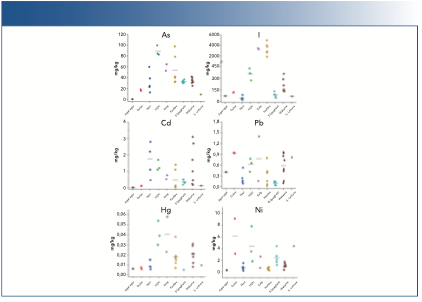
Heavy metals results show that mercury and nickel are not a threat in seaweed consumption because their contribution to the TDI was minimal (<1% and <6% for mercury and nickel, respectively). Regarding cadmium and lead, their contribution may be significant in some specific seaweeds. In some nori and wakame samples, a portion of 5 g could provide up to 70% of the allowed daily intake of cadmium. On the other hand, some kelp, wakame, and dulse samples presented a sizable contribution of lead, between 15–23%.
Concerning total arsenic, values obtained ranged from 0.4 mg/kg to 100 mg/kg. However, inorganic arsenic results were much lower in most of the samples. In nori, kelp, and sea spaghetti samples, inorganic arsenic could not be determined (< 0.10 mg/kg). The rest of the species other than hijiki had inorganic arsenic values close to the quantification limit, and their contribution to the TDI was not significant (<16%). Regarding hijiki, it must be highlighted the average of inorganic arsenic found was 62 mg/kg, much higher than the rest of species. This amount exceeds the corresponding TDI in up to 1780%, which means hijiki’s inorganic arsenic content may pose to a risk to consumers if ingested regularly.
Because both total arsenic and inorganic arsenic were quantified, it is possible to estimate the contribution of the inorganic fraction to the total. For hijiki seaweed, 70% of the total arsenic is in inorganic form, an overwhelming percentage compared to other species. This could lead to the conclusion that this species does not metabolize arsenic the same way than other types of seaweed. Moreover, for agar-agar, the inorganic arsenic fraction is also important (45%), but the amount of total arsenic is low compared to the rest of the species examined (only 0.4 mg/kg).
Finally, iodine contributions were extraordinarily high in most cases. Only sea lettuce, agar-agar, and nori sample contributions were lower than 100% (up to 57%, 61%, and 72%, respectively). On the other hand, a 5 g portion of kelp or kombu exceeded the tolerable daily intake by approximately 3000 and 4000%, respectively, which could mean that an important potential risk exists in consuming kelp and kombu. In addition, EFSA published afterwards that an adequate intake for adults was 150 μg/day, lower than the upper TDI used in this study, which suggests a worse picture for the risk assessment.
Conclusions
The method has proved to be suitable in analyzing heavy metals, arsenic, and iodine in dried seaweed simultaneously in a unique sample pretreatment, over- coming iodine determination issues using ICP-MS.
A 5 g daily portion of seaweeds like agar-agar, dulse, nori, sea spaghetti and wakame does not present a critical risk to consumers. However, a decent amount of cadmium, lead, and iodine may be found in some of them.
Sea lettuce contain the lowest values of metals, arsenic, and iodine. However, only one sample was included in this study, so clear conclusions cannot be reached.
Inorganic arsenic content in hijiki seaweed were extraordinarily high in contrast to other seaweeds. According to the results of this study, it is recommended that humans only consume 2.5 g per week of hijiki seaweed.
Kelp and kombu species were found to contain extremely high concentrations of iodine. Considering the results obtained, an intake of no more than 1 g per week of these seaweeds is suggested; regular consumption of higher amounts may lead to iodine excess disorders in consumers.
References
(1) A.H. Buschmann, C. Camus, J. Infante, A. Neori, A. Israel, M..C. Hernández-González, S.V. Pereda, J.L. Gómez-Pinchetti, A. Golberg, N. Tadmor-Shalev, and A.T. Critchley, Eur. J. Phycol. 52(4), 391–406 (2017).
(2) E.M. Brown, P.J. Allsopp, P.J. Magee, C.I. Gill, S. Nitecki, C.R. Strain, and E.M. Mcsorley, Nutr. Rev. 72(3), 205–216 (2014).
(3) S.L. Holdt and S. Kraan, J. Appl. Phycol. 23(3) 543–597 (2011).
(4) P. MacArtain, C.I. R. Gill, M. Brooks, R. Campbell, and I.R. Rowland, Nutr. Rev. 65(12), 535–543 (2007).
(5) V. Quitral, C. Morales, M. Sepúlveda, and M. Schwartz, Rev. Chil. Nutr. 39(4), 196–202 (2012).
(6) J.L. Banach, E.F. Hoek-van den Hil, and H.J. van der Fels-Klerx, Compr. Rev. Food. Sci. Food. Saf. 19(2), 332–364 (2020).
(7) L. Jarüp, Br. Med. Bull. 68(1), 167–182 (2003).
(8) Z. Ma, L. Lin, M. Wu, H. Yu, T. Shang, T. Zhang, and M. Zhao, Aquac. 497(1), 49–55 (2018).
(9) V. F. Taylor and B. P. Jackson, Chemosphere 163(1), 6–13 (2016).
(10) M. Leermarkers, W. Baeyens, M. De Gieter, B. Smedts, C. Meert, H.C. De Bisschop, R. Morabito, and P.H. Quevauviller, Trends Anal. Chem. 25(1), 1–10 (2006).
(11) Scientific Committee on Food (SCF), Scientific Panel on Dietetic Products, Nutrition and Allergies. Tolerable Upper Intake Levels for Vitamins and Minerals (2006). https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf.
(12) M.B. Zimmerman, Y. Ito, S.Y. Hess, K. Fujieda and L. Molinari, Am. J. Clin. Nutr. 81(4), 840–844 (2005).
(13) M.B. Zimmerman, Endocr. Rev. 30(4), 376–408 (2009).
(14) A. Lähteenmäki-Uutela, M. Rahikainen, M.T. Camarena-Gómez, J. Piiparinen, K. Spilling, and B. Yang, Aquac. Int. 29(2), 487–509 (2021).
(15) Commission Regulation (EC) No 629/2008 of 2 July 2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008R0629.
(16) Commission Recommendation (EU) 2018/464 of 19 of March 2018 on the monitoring of metals and iodine in seaweed, halophytes and products based on seaweed. https://eur-lex.europa.eu/legal-content/EN/TXT/uri=uriserv%3AOJ.L_.2018.078.01.0016.01.ENG.
(17) M.S. Reid, K.S. Hoy, J.R.M. Schofield, J.S. Uppal, Y. Lin, X. Lu, H. Peng, X.C. Le, Trends Anal. Chem. 123(1), 115770 (2020)
(18) H.J. Reid, A.A. Bashammakh, P.S. Goodall, M.R. Landon, C. O ́Connor, and B.L. Sharp, Talanta 75(1), 189–197 (2008).
(19) E.M.M. Flores, P.A. Mello, S.R. Krzyaniak, V.H. Cauduro, and R.S. Picoloto, RCM 34(S3), e8727 (2020). DOI: 10.1002/rcm.8727.
(20) K. Fujisaki, H. Matsumoto, Y. Shimokawa, and K. Kiyotaki, Anal. Sci. 32(2), 167–170 (2016).
(21) T.T. Zava and T.D. Zava, Thyroid. Res. 4(14) (2011).
(22) M.Y. Roleda, H. Marfaing, N. Desnica, R. Jóns-dóttir, J. Skjermo, G. Rebours, and U. Nitschke, Food. Control 95(1), 121–134 (2019).
(23) EFSA (European Food Safety Authority), EFSA Journal 7(3), 980 (2009).
(24) EFSA, EFSA Journal 18(11), 6268 (2020).
(25) EFSA, EFSA Journal 10(12), 2985 (2012).
(26) EFSA, EFSA Journal 8(4), 1570 (2010).
(27) EFSA, EFSA Journal 7(10), 1351 (2009).
Javier Peinador Asensio is with the Head División at the Laboratorio de Salud Pública de Madrid Salud in Madrid, Spain. Daniel Arnaiz Uceda is with the Technical Laboratory at the Laboratorio de Salud Pública de Madrid Salud in Madrid, Spain. Pilar Jiménez Navarro is an Assistant to the Department at the Laboratorio de Salud Pública de Madrid Salud in Madrid, Spain. Direct correspondence to: peinadoraj@madrid.es.

Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.
Best of the Week: Hyperspectral Imaging, ICP-MS Analysis of Geological Samples, Product Roundup
October 18th 2024Top articles published this week include an article about hyperspectral imaging in human skin research, a peer-reviewed article about analyzing geological samples using atomic spectroscopy techniques, and an equipment roundup piece about the latest products in the industry.