Vibrational Spectroscopic Analysis of Six New Hexafluorosilicate Salts Containing Different Amino Acids
Vibrational spectra of six new hexafluorosilicate salts (b-AlaH)(BetH)SiF6·H2O (I), (β-AlaH)(l-ProH)SiF6 (II), (β-AlaH)(l-ProH···l-Pro)SiF6 (III), (BetH)(BetH···Sar)SiF6·H2O (IV), (GlyH···DMG)2SiF6 (V), and (DMGH···Sar)2SiF6 (VI), containing different amino acids (glycine (Gly), sarcosine (Sar), dimethylglycine (DMG), betaine (Bet), β-alanine (β-Ala) and l-proline (l-Pro)) are typically recorded and discussed on the basis of their structures. The presence of cations, dimeric cations with short hydrogen bonds, and hexafluorosilicate anions is reflected in the spectra.
Amino acids can form salts of various types (1). In particular, Rajaram’s group has reported salts with anionic sublattice composed of different anions: (l-LysH2···l-LysH)(Cl)2(ClO4), (l-LysH2···l-LysH)(Cl)2(NO3), and (l-OrnH2)2(Cl)(NO3)(SO4) (2–4). Later new salts with mixed anions containing glycine, sarcosine, l-histidine and l-arginine (5–12) were obtained. Mixed salts with different anions represent an interesting class of compounds from the crystal chemistry point of view, as well as from the perspective of new materials for various applications. Recently, we discovered a new class of salts that contain two different amino acids (13–16). We have already reported crystal structures of four types of salts containing hexafluorosilicate anion and the following amino acids: glycine (Gly), sarcosine (Sar), dimethylglycine (DMG), betaine (Bet), β-alanine (β-Ala) and l-proline (l-Pro) (16). These salts were grouped into four categories according to the general formulae: 1. A(1)+A(2)+Y2- ‒ (β-AlaH)(BetH)SiF6·H2O (I) and (β-AlaH)(l-ProH)SiF6 (II), 2. A(1)+[A(2)+×××A(2)]Y2- ‒ (β-AlaH)(l-ProH···l-Pro)SiF6 (III), 3. A(1)+[A(1)+×××A(2)]Y2- ‒ (BetH)(BetH···Sar)SiF6·H2O (IV), and 4. [A(1)+×××A(2)]2Y2- ‒ (GlyH···DMG)2SiF6 (V) and (DMGH···Sar)2SiF6 (VI) (16).
Simple salts containing hexafluorosilicate anion and amino acid cations, as well as other cations, are known to have anti-caries activity (17–29).
In the present work, the vibrational spectra of the salts (I–VI) are analyzed based on their crystal structures. For the free XY6 octahedral molecule, there are six normal modes of vibration: ν1, ν2 and ν5 are Raman-active, ν3 and ν4 are infrared-active, and deformation ν6 is inactive both in the Raman and infrared spectrum (30). Thus, the free octahedral anion (SiF6)2- is characterized by the following normal modes of vibrations: ν1 (663 cm-1), ν2 (477 cm-1), ν5 (408 cm-1) (Raman active), and ν3 (741 cm-1), ν4 (483 cm-1) (both IR-active) (27). If the anion is isolated, these vibrations should appear as single bands. In other words, the free anion (SiF6)2- has Oh symmetry, and the internal vibrational modes correspond to the following irreducible representations: 1A1g(R)+1Eg(R)+1F2g(R)+2F1u(IR)+1F2u (inactive in IR and Raman). Because of intermolecular interactions, the ion symmetry in the crystal becomes lower than the symmetry in the gaseous state. As a consequence, the octahedral ions XY6 exhibit splitting of degenerate vibrations and some IR-active modes become Raman-active, while Raman-active modes become IR-active, accordingly to their site-symmetries in the corresponding crystal structures space group (30–32).
For an analysis of the vibrational bands in the spectra of crystals, it is necessary to do a site group (factor group) analysis. Halford listed all possible site symmetries and the number of equivalent sites for all 230 space groups (33). The internal vibrational modes of the SiF62- anion are described in detail in several works (34–39).
Materials and Methods
For initial reagents, we used β-alanine (≥99% NT), betaine monohydrate (≥98%), l-proline (≥99% FCC, FG), sarcosine (≥98%), and hexafluorosilicic acid (34%) purchased from Sigma-Aldrich Co. N,N-dimethylglycine hemihydrate (≥98.0%) purchased from Shanghai Worldyang Chemical Co. Crystals of salts (I‒VI) have been obtained by slow evaporation at room temperature from aqueous solution containing components in stoichiometric ratios: 1:1:1 [(β-AlaH)(BetH)SiF6·H2O (I)]; 1:1:1 [(β-AlaH)(l-ProH)SiF6 (II)]; 1:2:1 [(β-AlaH)(l-ProH···l-Pro)SiF6 (III)]; 2:1:1 [(BetH)(BetH···Sar)SiF6.H2O (IV)]; 2:2:1 [(GlyH···DMG)2SiF6 (V) and (DMGH···Sar)2SiF6 (VI)]. To prepare these solutions, the following mixtures were dissolved in 25 mL of H2O in polypropylene vessels: (I) - 1.000 g of β-alanine, 1.517 g of betaine monohydrate and 4.756 g of hexafluorosilicic acid; (II) - 1.000 g of l-proline, 0.774 g of β –alanine and 3.681 g of hexafluorosilicic acid; (III) - 1.000 g of l-proline, 0.387 g of β–alanine and 1.840 g of hexafluorosilicic acid; (IV)- 0.500 g sarcosine, 1.517 g of betaine monohydrate and 2.378 g of hexafluorosilicic acid; (V) - 1.000 g of glycine, 1.493 g of N,N-dimethylglycine and 5.643 g of hexafluorosilicic acid; (VI) - 1.000 g of sarcosine, 1.256 g of N,N-dimethylglycine and 4.757 g of hexafluorosilicic acid. The crystals were obtained by slow evaporation of these aqueous solutions at room temperature.
Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR FT-IR) were registered at room temperature by a LUMOS FT-IR Microscope (germanium prism, Happ-Genzel apodization, ATR distortion is corrected, number of scans 32; resolution: 4 cm-1). Part of the IR spectrum in the region 500–400 cm-1 were taken from FT-IR spectra registered by Avatar System 330 with Nujol mull technique (4000–400 cm-1, number of scans 32, resolution 2 cm-1). Fourier-transform Raman spectra were registered by an XploRA Raman Microscope from HORIBA Scientific at room temperature.
Some Structural Data
All known hexafluorosilicate salts of l-histidine (l-His) with different anions and salts containing different amino acids with their chemical formula, space group, number of formula units in the unit cell (Z), number of formula units in the asymmetric unit (Z'), and the respective references, are listed in Table I.
Results
4.1 Vibrational Spectra of (β-AlaH)(BetH)SiF6·H2O (I) and (β-AlaH)(l-ProH)SiF6 (II)
The vibrational spectra of (β-AlaH)(BetH)SiF6·H2O (I) and (β-AlaH)(l-ProH)SiF6 (II) are shown in Figure 1. The wavenumbers and tentative assignment of the peaks are listed in Table IS. In the IR spectrum of (β-AlaH)(BetH)SiF6·H2O (I) the broad absorption band at 3499 cm-1 corresponds to the ν(OH) stretching modes of H2O molecules. The peak at 1669 cm-1 is assigned to the deformation vibration of H2O molecules. The band with peaks at 3244 and 3199 cm-1 is caused by ν(NH) of the NH3+ groups and νOH) mode of COOH groups of b-alaninium and betainium cations. This band is superimposed with νCH) of CH3 and CH2 groups. The asymmetric unit cell contains three formula units, and therefore, there are six independent cations. The bond lengths of carboxyl groups are almost in the same ranges: C1A-O1A (1.325(2) Å), C1A-O2A (1.197(2) Å), C1B-O1B (1.329(2) Å), C1B-O2B (1.2029(19) Å), C1C-O1C (1.322(2) Å), C1C-O2C (1.192(2) Å) (β-alaninium cations), and C1D-O1D (1.3265(19) Å), C1D-O2D (1.200(2) Å), C1E-O1E (1.324(2) Å), C1E-O2E (1.200(2) Å), C1F-O1F (1.318(2) Å), C1F-O2F (1.198(2) Å) (betainium cations) (16). The strong band at 1734 cm-1 and the overlapped band at 1751 cm-1 are assigned to ν(C=O) of carboxyl groups. The peak at 1607 cm-1 is assigned to asymmetric deformation vibration of NH3+ groups of β-alaninium cations and peaks at 1511 cm-1 and 1495 cm-1 are caused by the symmetric deformation vibration of NH3+ groups of β-alaninium cations. Stretching vibrations of C-H bonds are well-presented in the Raman spectrum as the bands with peaks at 3066, 3043, 2993, 2979, 2946, and 2926 cm-1.
FIGURE 1: (a) Infrared and (b) Raman vibrational spectra of (β-AlaH)(BetH) SiF ·H O (I) and (β-AlaH)(l-ProH)SiF (II).
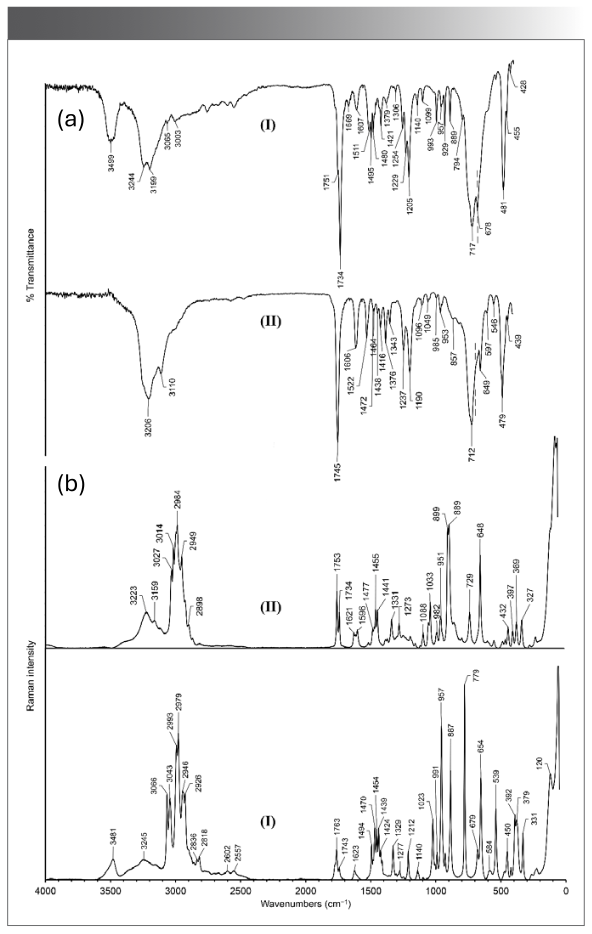
In the IR spectrum of (β-AlaH)(l-ProH)SiF6 (II) in the high-frequency region stretching modes of N-H, C-H and O-H bonds are expected. The characteristic band at 1745 cm-1 is assigned to ν(C=O). The band with a peak at 1606 cm-1 is due to the asymmetric deformation vibration of the NH3+ groups and the deformation vibration of the NH2+ groups. The peak at 1522 cm-1 is probably caused by symmetric deformation of NH3+ groups and peaks at 1464 cm-1, 1438 cm-1, and 1416 cm-1 by the deformation vibrations of CH2 groups. The peak at 1237 cm-1 is assigned to ν(C-OH). In (β-AlaH)(l-ProH)SiF6 (II), two anions are in general position and stabilized due to hydrogen bonds formed by the O-H and N-H groups with fluorine atoms. The Raman lines at 3223 cm-1 and 3159 cm-1 originate in stretching vibrations of N-H bonds of NH3+ and NH2+ groups, involved in the hydrogen bonds. The similar broad band is also observed in the IR spectrum at 3206 cm-1. The presence of CH and CH2 groups is confirmed by characteristic Raman lines at 3027, 3014, 2984, 2949, and 2898 cm-1.
Vibrational Modes of SiF62- Anions
The (β-AlaH)(BetH)SiF6·H2O (I) crystal has P21/n symmetry (Z=12, Z'=3): there are three crystallographically different anions in the general position. The fluorine atoms F2, F11, and F15 do not form hydrogen bonds at all, while other fluorine atoms are involved at least in one hydrogen bond (16). The SiF62- anions are ordered, and their Si–F distances are in the range of 1.6509(10) to 1.6968(10) Å (16). The anions can be described as slightly distorted. In the crystal (C2h factor group symmetry) the SiF62- anions have C1 site symmetry. Because of lowering symmetry, Raman-active ν1(SiF62-) and ν2(SiF62-) modes became active in IR (respectively, at 678 cm-1 and 455 cm-1). The strong absorption band at 717 cm-1 is the most characteristic indication of the hexafluorosilicate anion caused by asymmetric stretching ν3 mode, which is close to 720 cm-1, as observed in the spectrum of #2 (Table I). Another Raman active mode ν5 (392 cm-1, 379 cm-1) is also observed in the IR spectrum (428 cm-1).
In the structure of (β-AlaH)(l-ProH)SiF6 (II) (P21, Z=4, Z'=2), there are two crystallographically different anions in the general position. The fluorine atoms are crystallographically independent, and due to various strong hydrogen bonds formed by the O-H and N-H groups with fluorine atoms, the hexafluorosilicate anions are distorted from their octahedral geometries (Si–F distances range from 1.6530(14) to 1.7026(13) Å [16]). In the crystal (C2 factor group symmetry), the SiF62- anions have C1 site symmetry. As in the case of (β-AlaH)(BetH)SiF6·H2O (I), all Raman-active modes appear in the IR spectrum, and vice versa. The broad strong band observed in the IR spectrum at 712 cm-1 and in the Raman spectrum at 729 cm-1 is assigned to IR-active n3 asymmetric stretching mode of hexafluorosilicate anions. This is close to 715 cm-1 observed in the spectrum of #5 (Table I). There is also a well-noticed band at 649 cm-1, which is assigned to usually Raman-active ν1 modes of anions. This assumption is supported by the Raman line at 648 cm-1. The latter appears due to the distortion of the octahedral anions and their symmetry lowering from Oh in the free state to C1 in the crystal.
4.2 Vibrational Spectra of (β-AlaH)(l-ProH···l-Pro)SiF6 (III) and (BetH)(BetH···Sar)SiF6·H2O (IV)
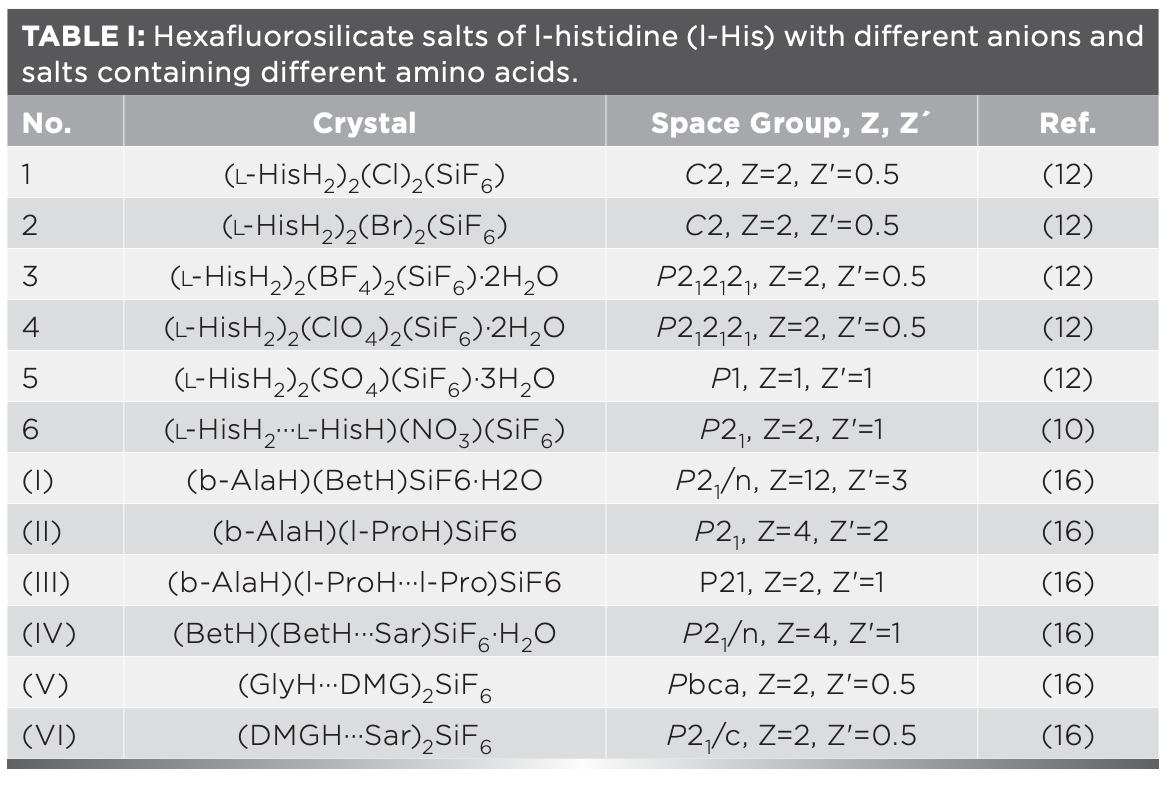
The vibrational spectra of (β-AlaH)(l-ProH···l-Pro)SiF6 (III) and (BetH)(BetH···Sar)SiF6·H2O (IV) are shown in Figure 2. The wavenumbers and tentative assignment of the peaks are listed in Table IIS. Bond lengths of carboxyl groups in β-alaninium cation C1A-O1A = 1.322(3) Å and C1A-O2A = 1.169(3) Å, while those in l-prolinium cation C1B-O1B = 1.289(2) Å) and C1B-O2B = 1.221(2) Å (16). In the structure of (β-AlaH)(l-ProH···l-Pro)SiF6 the dimeric cation (l-ProH···l-Pro) is characterized by a strong O1B-H1B···O2C hydrogen bond with a short O···O distance of 2.438(2) Å. For strong hydrogen bonds (R(O···O) = 2.40‒2.58 Å), the correlation of ν(OH)(cm-1) vs. R(O···O) (Å) (39) has approximately linear character: ν(OH)=12500R-29875. The expected position of ν(OH) based on this correlation for the R(O···O) distance of 2.438(2) Å is ca. 600 cm-1. In the spectrum of (III) in the region 1000-400 cm-1, one can notice a broad band centered at 700 cm-1. The carboxyl group of β-alaninium cation forms a O1A-H1A···F4 hydrogen bond with the anion where the distances H1A···F4 and O1A···F4 are 1.87 and 2.678 Å, respectively. Based on this, the broad IR absorption band at 3193 cm-1 and the Raman line at 3189 cm-1 can be assigned to ν(OH) of β-alaninium cation and ν(NH) of NH3+ group. Stretching vibrations of C–H bonds are well visible in the Raman spectrum as the bands at 3037, 2994, 2970, 2953 and 2885 cm-1. The corresponding vibration modes are not observed in the IR spectrum, excluding the weak band at 2995 cm-1.
FIGURE 2: (a) Infrared and (b) Raman vibrational spectra of (β-AlaH)(l- ProH···l-Pro)SiF6 (III) and (BetH)(BetH···Sar)SiF6·H2O (IV).

The characteristic bands with peaks at 1734 cm-1 and 1675 cm-1 are assigned to ν(C=O) of β-alaninium and l-prolinium cations, respectively. Characteristic bands at 1606 cm-1 and 1565 cm-1 are assigned to asymmetric deformation of NH3+ and deformation vibration of NH2+ groups, respectively. The band with a peak at 1517 cm-1 is caused by symmetric deformation vibrations of the NH3+ group of β-alaninium cation.
In the structure of (BetH)(BetH···Sar)SiF6·H2O(IV), the asymmetric unit contains one formula unit. The dimeric cation (BetH···Sar) is formed through the hydrogen bond O1B-H1B···O1A between the betainium cation and the zwitterionic sarcosine with O···O distance of 2.5310(12) Å. Another betainium cation forms a hydrogen bond with the H2O molecule O1C-H1C···O1W with O···O distance equal to 2.5778(15) Å (16). The presence of water molecules is reflected by a characteristic band at 3567 cm-1 (and a Raman line at 3569 cm-1). The IR band at 3193 cm-1 is assigned to ν(NH) of the NH2+ group (corresponding Raman line at 3191 cm-1). Raman lines at 3057–2969 and respective peaks in the IR spectrum at 3050–3005 are assigned to ν(CH) of CH3 and CH2 groups.
In the spectrum of (IV), there is a broad absorption centered at ca. 2500 cm-1, which is absent in the spectrum of (III). This absorption band correlates well with the vibration ν(OH) expected for the O1C-H1C···O1W hydrogen bond. The expected value of ν(OH) for the carboxyl group of betainium in the (BetH···Sar) dimer is ca. 1760 cm-1. There is no pronounced absorption in this region, but a broad band can be seen in the 2100–1330 cm-1 region centered near the expected value. The band with a peak at 1748 cm-1 is assigned to ν(C=O) of the betainium cation connected to the water molecule, while the band with a peak at 1685 cm-1 may be caused by ν(C=O) of the betainium cation in the (BetH···Sar) dimer and by the deformation vibration of water molecule.
Vibrational Modes of SiF62- Anions
In (β-AlaH)(l-ProH···l-Pro)SiF6 (III), the hexafluorosilicate anion occupies a general position (P21, Z=2, Z'=1). Si–F bonds have almost the same lengths (Si–F distances range from 1.661(2) to 1.6831(16) Å [16]). The crystal factor group symmetry is C2 and the SiF62- anion site symmetry is C1. Despite the symmetry lowering, the IR active modes (ν 3 andν 4) do not become active in the Raman spectrum, most likely because of small distortion of the octahedral. The peak at 653 cm-1 (and the Raman line at 650 cm-1) were assigned to ν 1 of SiF62-, similar to the absorption band at 649 cm-1 in (β-AlaH)(l-ProH)SiF6 (II). The characteristic ν 3 mode of SiF62- is observed at 720 cm-1, as in #2 (Table I). The well-visible band at 479 cm-1 is assigned to the ν 4 mode of the hexafluorosilicate anion.
The (BetH)(BetH···Sar)SiF6·H2O (IV) crystal has P21/n symmetry (Z=4, Z'=1). The hexafluorosilicate anion is situated in the general position. The factor group of the crystal is C2h and the SiF62- anion has C1 site symmetry. The hexafluorosilicate anion symmetry is not far from Oh, since the Si–F distances range only from 1.6667(9) to 1.6863(9) Å (16). The characteristic ν3 mode is observed at 713 cm-1, which is close to the respective value 715 cm-1 of #5 (Table I). The peak at 662 cm-1 assigned to ν1 of SiF62- and the Raman line at 647 cm-1 are less pronounced. The strong absorption band at 475 cm-1, which is absent in the Raman spectrum, is assigned to the second IR active ν4 mode of SiF62- anion.
4.3. Vibrational Spectra of (GlyH···DMG)2SiF6 (V) and (DMGH···Sar)2SiF6 (VI)
The vibrational spectra of (GlyH···DMG)2SiF6 (V) and (DMGH···Sar)2SiF6 (VI) are shown in Figure 3. Wavenumbers and tentative assignment of the peaks are listed in Table IIIS. The asymmetric unit of (GlyH···DMG)2SiF6 (V) contains half of the formula unit (16). The O···O distance in the dimeric (GlyH···DMG) cation is 2.4980(9) Å. The NH3+ group of the glycinium cation forms hydrogen bonds with the hexafluorosilicate anion. The IR broad band centered at 3142 cm-1 and the Raman lines at 3191 cm-1 are caused by ν(NH) of the NH3+ and NH+ groups of the dimethylglycine molecule, while the peaks at 3039 cm-1, 3021 cm-1, 2970 cm-1, and 2850 cm-1 (and the corresponding Raman lines at 3057-2820 cm-1) are assigned to the stretching vibration of C–H bonds of CH2 and CH3 groups. The characteristic band at 1718 cm-1 and the Raman line at 1736 cm-1 are assigned to ν(C=O) of the glycinium cation. In the spectrum of (V), the peaks in the 1800–900 cm-1 region are superimposed with a broad absorption centered at ca. 1400 cm-1. This is well-correlated with the expected value, 1350 cm-1, of ν(OH) for R(O···O) = 2.4980(9) Å.
FIGURE 3: (a) Infrared and (b) Raman vibrational spectra of (GlyH···DMG)2SiF6 (V) and (DMGH···Sar)2SiF6 (VI).

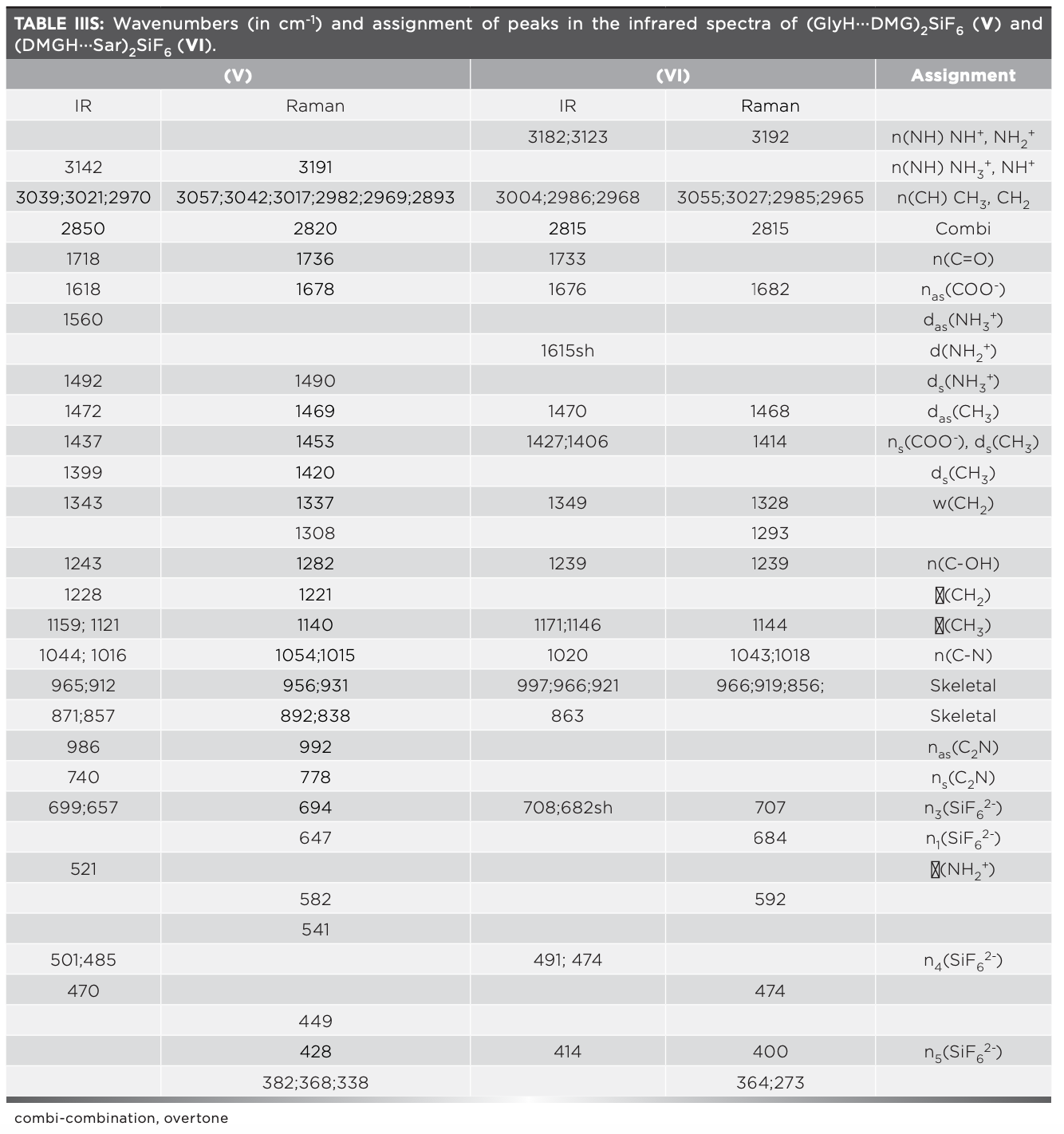
The asymmetric unit of (DMGH···Sar)2SiF6 (VI) also contains half of formula unit. In the dimer (DMGH···Sar), the hydrogen atom is almost in the middle position (O1B-H1A = 1.29 Å, H1A-O1A = 1.21 Å and O1A-H1A···O1B = 2.4610(19 Å)). The C–O distances are as follows: C1A-O1A = 1.269(2) Å) and C1A-O2A = 1.226(2) Å) in zwitterionic sarcosine, while in dimethylglycinium cation, C1B-O1B = 1.287(2) Å) and C1B-O2B = 1.220(2) Å (16). The characteristic band at 1733 cm-1 is assigned to ν(C=O) of the dimethylglycinium cation and that at 1676 cm-1 to νas(COO-) of the carboxylate group of zwitterionic sarcosine. The shoulder at 1615 cm-1 is assigned to the deformation vibration of the NH2+ group of sarcosine. In the IR spectrum, the bands at 3182 cm-1 and 3123 cm-1 (and the Raman line at 3192 cm-1) are caused by ν(NH) of the NH+ group of dimethylglycinium cation and the NH2+ group of zwitterionic sarcosine. The peaks at 3004 cm-1, 2986 cm-1, and 2968 cm-1 (and the Raman lines at 3055 cm-1, 3027 cm-1, 2985 cm-1 and 2968 cm-1) are caused by stretching vibration of the C–H bonds of CH2 and CH3 groups. The O···O distance in (VI) is shorter than in (V), and the expected value of ν(OH) is ca. 900 cm-1 (40). Peaks in the 1500 cm-1–700 cm-1 region are superimposed with broad absorption centered at ca. 1100 cm-1. This is in correlation with the expected value of 900 cm-1 of ν(OH) for R(O···O) = 2.4610(19) Å.
Vibrational Modes of SiF62- Anions
The crystals (GlyH···DMG)2SiF6 (V) and (DMGH···Sar)2SiF6 (VI) have Pbca (Z=4, Z'=0.5) and P21/c (Z=2, Z'=0.5) symmetries, respectively. In both structures, SiF62- anions are in a special position and are located at the Ci sites in the crystal lattice. The factor groups of (GlyH···DMG)2SiF6 (V) and (DMGH···Sar)2SiF6 (VI) are D2h and C2h, respectively. For this reason, the ν1 Raman active mode cannot be active in IR. At the same time, by the factor group effect (Davydov splitting), ν3 and ν4 modes are split into two components. Splitting of the vibrational modes is expected for SiF62- in the crystal as a result of lowering the symmetry (Oh→ Ci). In the infrared spectrum of (GlyH···DMG)2SiF6 (V), the characteristic strong band at 699 cm-1 and the band at 657 cm-1 are assigned to the n3 mode of SiF62-. Similar bands were observed at 697 cm-1 and 698 cm-1 in the spectra of #3 and #4 (Table I). Two noticeable IR peaks observed at 485 cm-1 and 470 cm-1 are assigned to ν4(SiF62-). In the infrared spectrum of (DMGH···Sar)2SiF6 (VI), the strong band observed at 708 cm-1 and the band at 682 cm-1 are caused by ν3 asymmetric stretching mode, which are close to 706 cm-1, as observed in the spectrum of #6 (Table I). Two IR peaks at 491 cm-1 and 474 cm-1 are assigned to ν4(SiF62-).
Conclusion
Investigation of the vibrational spectra of hexafluorosilicate salts containing different amino acids (β-AlaH)(BetH)SiF6·H2O (I), (β-AlaH)(l-ProH)SiF6 (II), (β-AlaH)(l-ProH···l-Pro)SiF6 (III), (BetH)(BetH···Sar)SiF6·H2O (IV), (GlyH···DMG)2SiF6 (V) and (DMGH···Sar)2SiF6 (VI) allows us to draw some general conclusions. In all salts, the presence of hexafluorosilicate anions is confirmed by their most characteristic ν3 mode. The cations are visible through the characteristic bands of ν(C=O), and the crystallization water molecules in the structures of (I) and (IV) are reflected in the spectra by the presence of characteristic bands at 3487 cm-1 and 3575 cm-1, respectively. There are different cases related to hexafluorosilicate modes depending on the symmetry and degree of distortion. In (β-AlaH)(BetH)SiF6·H2O (I) and (β-AlaH)(l-ProH)SiF6 (II), all Raman-active modes appear in the IR spectra, and vice versa. In (β-AlaH)( l-ProH··· l-Pro)SiF6 (III) and (BetH)(BetH···Sar)SiF6·H2O (IV), there are no IR-active modes in Raman spectra, and vice versa, as they are absent or less pronounced. In (GlyH···DMG)2SiF6 (V) and (DMGH···Sar)2SiF6 (VI), IR-active modes and Raman-active modes appear only in the IR and Raman spectra, respectively.
Acknowledgments
This work was supported by the RA MES Committee of Science, in the frames of the research projects № 18T-1D033. G.S. Tonoyan also thanks the “PhD Support Program 2022,” which is implemented by the Enterprise Incubator Foundation with the support of PMI Science. I thank my supervisor, Dr. Aram Petrosyan, and Dr. Gerald Giester for their help and useful discussions.
References
(1) Fleck, M.; Petrosyan, A. M. Salts of Amino Acids: Crystallization, Structure and Properties; Springer, 2014.
(2) Srinivasan, N.; Sridhar, B.; Rajaram, R. K. Hydrogen bis[L-lysinium(2+) dichloride perchlorate. Acta Crystallogr. E 2001, 57 (9), o875–o877. DOI: 10.1107/S1600536801013484
(3) Srinivasan, N.; Sridhar, B., Rajaram, R. K. L-lysine L-lysinium dichloride nitrate. Acta Crystallogr. E 2001, 57 (9), o888–o890. DOI:10.1107/S1600536801014040
(4) Ramaswamy, S.; Sridhar, B.; Ramakrishnan, V.; Rajaram, R. K. Bis(L-ornithinium) chloride nitrate sulfate. Acta Crystallogr. E 2004, 60 (5), o768–o770. DOI: 10.1107/S1600536804007238
(5) Shtemenko, A. V.; Kozhura, O. V.; Pasenko, A. A.; Domasevitch, K. V. New Octachlorodirhenate(III) Salts: Solid State Manifestation for a Certain Conformational Flexibility of the [Re2Cl8]2- Ion. Polyhedron 2003, 22 (12), 1547–1552. DOI: 10.1016/s0277-5387(03)00288-2
(6) Giester, G.; Ghazaryan, V. V.; Fleck, M. et al. Mixed Salt of Sarcosine Containing Dimeric Undecafluoridodialuminate Anion and Fluoride Ion. J. Fluor. Chem. 2018, 209, 73–78. DOI: 10.1016/j.jfluchem.2018.02.011
(7) Petrosyan, A. M; Fleck, M.; Ghazaryan, V. V. Mixed Salts of Amino Acids: Syntheses, Crystal Structure and Vibrational Spectra of L-histidinium(2+) Nitrate-perchlorate and L-histidinium(2+) Nitrate-tetrafluoroborate. Z. Kristallogr. 2010, 225 (9), 388–395. DOI: 10.1524/zkri.2010.1269
(8) Ghazaryan, V. V.; Fleck, M.; Petrosyan, A. M. Mixed Salts of Amino Acids: On the Vibrational Spectra of Mixed Salts Containing a L-lysinium(+)...L-lysinium(2+) Dimeric Cation. J. Mol. Struct. 2010, 982 (1–3), 145–151. DOI: 10.1016/j.molstruc.2010.08.020
(9) Ghazaryan, V. V.; Fleck, M.; Petrosyan, A. M. Mixed Salts of Amino Acids: L-lysinium(2+) Chloride Nitrate, L-lysinium(2+) Chloride Tetrafluoroborate and L-lysinium(2+) Chloride Perchlorate. J. Mol. Struct. 2010, 984 (1-3), 268–275. DOI: 10.1016/j.molstruc.2010.09.039
(10) Ghazaryan, V. V.; Fleck, M.; Petrosyan, A. M. Mixed Salts of Amino Acids: L-histidinium(+)...L-histidinium(2+) Nitrate-hexafluorosilicate. J. Mol. Struct. 2012, 1026, 140–144. DOI: 10.1016/j.molstruc.2012.05.052
(11) Ghazaryan, V. V.; Fleck, M.; Petrosyan, A. M. Mixed Salts of Amino Acids with Different Anions. J. Cryst. Growth 2013, 362 (1), 182–188. DOI: 10.1016/j.jcrysgro.2011.09.059
(12) Petrosyan, A. M.; Fleck, M.; Ghazaryan, V. V. New Mixed Salts of L-histidinium(2+) Comprising Hexafluorosilicate Anion. J. Cryst. Growth 2014, 401, 863–868. DOI: https://doi.org/10.1016/j.jcrysgro.2013.10.060
(13) Petrosyan, A. M.; Giester, G.; Tonoyan, G. S.; Ghazaryan, V.V.; Fleck, M. A New Class of Amino Acids Salts. In Book of Abstarcts, 3rd German Polish Conference on Crystal Growth (GPCCG-3), March 17-21, 2019, Poznań, Poland, p. 57. http://gpccg2019.put.poznan.pl/wp-content/uploads/2019/03/ABSTRACTS_ONLINE.pdf
(14) Petrosyan, A. M.; Giester, G.; Tonoyan, G. S.; Ghazaryan, V.V.; Fleck, M. Salts Containing Different Amino Acids: Three Types Salts of L-arginine Containing Glycine, Dimethylglycine or β-alanine. J. Mol. Struct. 2021, 1228, 129717. DOI: 10.1016/j.molstruc.2020.129717
(15) Tonoyan, G. S.; Giester, G.; Petrosyan, A. M. Salts Containing Different Amino Acids: Salts with β-alaninium L-proline Dimeric Cation. J. Mol. Struct. 2022, 1252, 132171. DOI: 10.1016/j.molstruc.2021.132171
(16) Petrosyan, A. M.; Giester, G.; Tonoyan, G. S.; Fleck, M. Salts Containing Different Amino Acids: Four Types of Salts with Hexafluorosilicate Anion. Struct. Chem. 2023, 34 (2), 491–504. DOI: 10.1007/s11224-022-01980-6
(17) Fleck, M.; Ghazaryan, V. V.; Petrosyan, A. M. Hexafluorosilicates of Sarcosine, Solid State Sci. 2012, 14 (7), 952–963. DOI: 10.1016/j.solidstatesciences.2012.04.036
(18) Ghazaryan, V. V.; Fleck, M.; Petrosyan, A.M. Hexafluorosilicates of Alanine, Z. Kristallogr. 2012, 227 (9), 646–655. DOI: 10.1524/zkri.2012.1537
(19) Ghazaryan, V. V.; Fleck, M.; Petrosyan, A. M. Salts of Amino Acids with Hexafluorosilicate Anion, J. Cryst. Growth 2013, 362 (1), 162–166. DOI: 10.1016/j.jcrysgro.2011.11.017
(20) Fleck, M.; Ghazaryan, V. V.; Petrosyan, A. M. Amino Acid Hexafluorosilicates – An Overview. Z. Kristallogr. 2013, 228 (5), 240–249. DOI: 10.1524/zkri.2013.1604
(21) Ghazaryan, V. V.; Fleck, M.; Petrosyan, A. M. New Chemical Analogs of Triglycine Sulfate. J. Cryst. Growth 2014, 401, 857–862. DOI: 10.1016/j.jcrysgro.2013.11.054
(22) Gelmboldt, V. O.; Kravtsov, V. Ch.; Fonari, M. S. Ammonium Hexafluoridosilicates: Synthesis, Structures, Properties, Applications. J. Fluor. Chem. 2019, 221, 91–102. DOI: 10.1016/j.jfluchem.2019.04.005
(23) Ouasri, A.; Rhandour, A. Structural, Vibrational, Thermal, Phase Transitions, and Properties Review of Alkylammonium, Alkylenediammonium, and Aminoacid Hexafluorosilicate Salts. Russ. J. Coord. Chem. 2021, 47 (7), 502–517. DOI: 10.1134/S1070328421070046
(24) Petrosyan, A. M.; Ghazaryan, V. V.; Fleck, M.; et al. Hexafluorosilicates of Amino Acids Having Anti-Caries Activity. Armenian patent #2695, 2012.
(25) Brsikyan, N. A.; Andriasyan, L. H.; Badalyan, G. R.; et al. Comparative Morphology of Dentinal Tubules Occlusion at the Use of Different Desensitizing Agents in Experiment. New Armen. Med. J. 2012, 6 (4), 52–55. https://ysmu.am/v2/wp-content/uploads/2023/09/7-Brsikyan-Inter-NAMJ-v6n4-Eng.pdf
(26) Gelmboldt, V. O.; Anisimov, V. Yu.; Shyshkin, I. O.; Fonari, M. S.; Kravtsov, V. Ch. Synthesis, Crystal Structures, Properties and Caries Prevention Efficiency of 2-, 3-, 4-Carboxymethylpyridinium Hexafluorosilicates. J. Fluor. Chem. 2018, 205, 15–21. DOI: 10.1016/j.jfluchem.2017.11.004
(27) Gelmboldt, V. O.; Shyshkin, I. O.; Anisimov, V. Yu.; Fonari, M. S.; Kravtsov, V. Ch. Bis(3-hydroxymethylpyridinium) Hexafluorosilicate Monohydrate as a New Potential Anticaries Agent: Synthesis, Crystal Structure and Pharmacological Properties. J. Fluor. Chem. 2020, 235, 109547. DOI: 10.1016/j.jfluchem.2020.109547
(28) Lytvynchuck, I. V.; Hritsiuk, A. H.; Gelmboldt, V. O. Synthesis, Structure and Some Properties of 2-, 3-, 4- Aminophenylacetic Acids Hexafluorosilicates. Issues Chem. Chem. Technol. 2022, 5, 63-68. DOI: 10.32434/0321-4095-2022-144-5-63-68
(29) Gelmboldt, V. O.; Lytvynchuk, I. V.; Shyshkin, I. O.; et al. Bis(2-, 3-, 4-carboxyethylpyridinium) Hexafluorosilicates as Potential Caries Prophylactic Agents. Arch. Pharm. 2022, 355, 2200074. DOI:10.1002/ardp.202200074
(30) Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Part A, 6th ed. John Wiley and Sons, 2009.
(31) Jeghnou, H.; Ouasri, A.; Rhandour, A.; et al. A. Structural Phase Transition in (n-C4H9NH3)2SiF6: DSC and Raman Studies. J. Raman Spectrosc. 2003, 34 (2), 126–130. DOI: 10.1002/jrs.964
(32) Jalbout, A.F.; Ouasri, A.; Jeghnou, H.; Rhandour, A. Experimental and Theoretical Studies of Monoalkylammonium hexafluorosilicate [CH3(CH2)nNH3]2SiF6 (n = 2, 3) and Ethylammonium Hexafluorosilicate [(C2H5)2NH2]2SiF6. Vib. Spectrosc. 2007, 44 (1), 94–100. DOI: 10.1016/j.vibspec.2006.09.006
(33) Halford, R. S. Motions of Molecules in Condensed Systems I. Selection Rules, Relative Intensities, and Orientation Effects for Raman and Infra-Red Spectra. J. Chem. Phys. 1946, 14 (1), 8–15. DOI: 10.1063/1.1724065
(34) Ouasri, A.; Lambarki, F.; Rhandour, A.; et al. Solid State DFT Modeling and Vibrational Characterisation of Butylenediammonium and Hexylenediammonium Hexafluorosilicate, NH3(CH2)nNH3SiF6 (n = 4 and 6). Vib. Spectrosc. 2017, 88, 83–93. DOI: 10.1016/j.vibspec.2016.11.006
(35) Ouasri, A.; Rhandour, A.; Dhamelincourt, M. C.; Dhamelincourt P.; Mazzah, A. Structural and Vibrational Study of Hydrazinium Hexafluorosilicate. J. Raman Spectrosc. 2002, 33 (9), 726–729. DOI: 10.1002/jrs
(36) Ouasri, A.; Rhandour, A.; Dhamelincourt, M. C.; et al. Infrared and Dielectric Studies of [(C2H5)4N]2SiF6, Phase Transit.: A Multinational J. 2003, 76 (7), 701–709. DOI: 10.1080/0141159021000037240
(37) Ouasri, A.; Rhandour, A.; Saadi, M.; El Ammari L. X-Ray, DSC, TGA-dTGA, and Vibrational Studies of the Propylenediammonium Hexafluorosilicate NH3(CH2)3NH3SiF6. Biointerface Res. Appl. Chem. 2021, 5 (11), 12618–12632. DOI: 10.33263/BRIAC115.1261812632
(38) Ouasri, A.; Rhandour, A.; Dhamelincourt, M.-C.; et al. Structural Phase Transitions in [(C2H5)4N]2SiF6: Differential Scanning Calorimetry and Raman Studies. J. Raman Spectrosc. 2002, 33 (9), 715–719. DOI: 10.1002/jrs.902
(39) Jeghnou, H.; Ouasri, A.; Elyoubi, M.; et al. Differential Scanning Calorimetric and Raman Studies of a Phase Transition in [C3H7NH3]2SiF6. J. Raman Spectrosc. 2004, 35 (4), 261–265. DOI: 10.1002/jrs.1145
(40) A. Novak, Vibrational Spectroscopy of Hydrogen Bonded Systems. In: Infrared and Raman Spectroscopy of Biological Molecules. NATO Advanced Study Institute Series, vol. 43, 1979, 279–303.
Gayane S. Tonoyan is with the Institute of Applied Problems of Physics at the National Academy of Sciences of Armenia, in Yerevan, Armenia. Direct correspondence to gayane.tonoyan@edu.isec.am or itonoyan1@gmail.com
Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.
Evaluation and Development Trends of Optical Detection Technology for Seed Vigor
In this article, the basic principles, advantages, and limitations of different optical techniques for obtaining seed vigor estimates are introduced and reviewed, and the key technology of non-destructive optical detection of single seeds will be discussed.