Agricultural and Environmental Management with Raman Spectroscopy
Spectroscopy
Raman spectroscopy is applied to quality control of agricultural products with greater frequency, and can also be used to refine regulatory criteria for both agricultural and environmental monitoring. It is now integrated into everything from hand held SERS detectors to unmanned aerial vehicles to monitor the gamut from genetic variation to soil and water content. Development opportunities, particularly with bundled technologies, continue to emerge as demand for quality assurance increases.
Raman spectroscopy has been applied to quality control of agricultural products with greater frequency, and it can also be used to refine regulatory criteria for both agricultural and environmental monitoring. Raman is now integrated into everything from handheld surface-enhanced Raman spectroscopy (SERS) detectors to unmanned aerial vehicles to monitor the gamut from genetic variation to soil and water content. Development opportunities, particularly with bundled technologies, continue to emerge as demand for quality assurance increases.
Raman spectroscopy applications have evolved for diverse disciplines including forensics (1), art and archeology (2), medicine (3), and agriculture (4,5). In 2013, the Agricultural Management Committee of the American Bar Association identified key areas in agriculture and the environment that would become an important focus for the legal community. These include national food safety issues, state genetically modified organism (GMO) labeling initiatives, air emissions from agriculture, and numeric nutrient criteria for the nation’s waters (6), among others. This highlights the growing need for accurate monitoring systems that are easy to use. An extensive review of Raman spectroscopy applied to a wide spectrum of agricultural issues by Yang and colleagues (5) discusses many of the agricultural applications for Raman spectroscopy in detail. In this article, current examples of Raman-bundled technologies, which exemplify future development opportunities, are cited for each of the key areas of emphasis identified by the Agricultural Management Committee, as summarized in Figure 1.
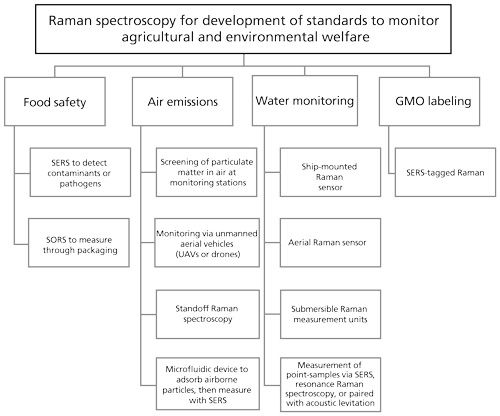
National Food Safety Issues
More than 15% of Americans become ill from food-borne diseases annually, causing 128,000 hospitalizations and 3000 deaths. Worldwide, more than one million deaths annually can be attributed to food and water-borne diarrheal diseases. Recent highly-publicized recalls of lettuce, tomatoes, peanut butter, milk, and dog food for various contaminants, and a recall of horse meat labeled as beef have decreased consumer confidence and trust. These, in addition to headlines for recalls of contaminated and mock dietary supplements, have highlighted the need for a rapid, accurate technology platform for controlling products. The practice of renaming and relabeling agricultural foodstuffs for profit remains loosely regulated without unifying international standards, and various imported and domestic contaminating factors, such as drugs or pathogens, can adversely affect native crops or potentially go undetected to be passed on for consumption (7–12). Therefore, development of technologies that facilitate rapid identification would be advantageous.
Advantages of Raman Spectroscopy
Raman spectroscopy offers a number of advantages compared to other analytical techniques. Gas chromatography (GC) or high performance liquid chromatography (HPLC) coupled to mass spectrometry (MS) is frequently used for chemical identification and polymerase chain reaction (PCR) is used for genetic identification. However, these methods require sending samples out to a laboratory where they must undergo rigorous preparation and sensitive assays performed by a highly skilled scientist. Additionally, other spectroscopic techniques may not be as well-suited for agricultural applications. For example, infrared (IR) spectroscopy is heavily influenced by water, whereas Raman spectroscopy is not (5).
Surface-enhanced Raman scattering (SERS) is very sensitive, requires minimal sample, and has a signal that is specific and stable (13). SERS uses metallized substrates or metal nanoparticles, typically silver or gold, to significantly reduce sample fluorescence and enhance Raman scattering by as much as 1014. Gold nanoparticle tagging provides stability and sensitivity for as long as 60 days post sample preparation, allowing for long-term monitoring (14). The benefits of using SERS to detect melamines, antibiotics, fungicides, pesticides, pathogens, and other contaminants in food sources has been discussed in depth (14). Some of the compounds successfully identified by SERS include pesticides that could be used in agricultural settings, but that have been deemed unsafe for human consumption. Many pathogens have been identified by SERS and include strains of Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, Salmonella typhimurium, Listeria monocytogenes, Enterococcus faecelis, Bacillus, Enterobacter, and various viridae including norovirus, adenovirus, parvovirus, simian rotavirus, coronavirus, Sendai virus, and herpes virus. These pathogens have been identified either alone or in mixtures, and it is postulated that single microorganisms or spores can be detected by SERS. Additionally, in some of the systems described, SERS accurately calculated the concentrations of the pathogens.
Spatially offset Raman spectroscopy (SORS) and inverse SORS use spatially offset regions of the sample for excitation and collection. This allows depth control of Raman measurement, including measurement of Raman spectra through packaging. This technique has been applied extensively for the detection of counterfeit pharmaceuticals and narcotics through sealed containers, including the identification of cocaine dissolved in alcohol (15). It can be easily modified for high-throughput detection of other adulterants in agricultural products, and potentially for meat source identification through manufacturer packaging.
In addition to the regulatory concerns with regard to national food safety issues, there is increasing interest in monitoring the nutritional value of foodstuffs for direct benefit to consumers based on their individual metabolic, genomic, and proteomic profiles in much the same way that pharmaceutical companies are delivering personalized medicine (16). Raman spectroscopy can inform directly and nondestructively on food nutrient profiles based on comparisons with desired quality standards, using only small amounts of sample (17).
Identification of Genetically Modified Organisms
Globally, as of 2011, there are reportedly 170 million hectares of cropland dedicated to GMOs with 69 million of these in the United States (18). There are strong arguments both for and against GMOs (19). Benefits such as decreased need for pesticide, higher yields to meet crop demands of an expanding global population, and skewed nutritional contents are polarized against fears of long-term effects on consumers, animals, and the environment. Farmers and consumers share concerns over the perceived concentration of power with the small consortium of agricultural biotechnology companies and their ability to withhold information because of patent protections (20). Most organisms are modified for a single trait, but not always. This includes resistance to pests, herbicides, or viruses, or some combination of these. The obvious benefit is the adaptation of crops to particular environments that would otherwise be unfruitful. Other applications include generating so-called “functional foods” that provide an additional benefit beyond basic nutrition by being enriched with a particular nutrient, or that offer some therapeutic benefit (20). In addition to nutritional context, there are also modifications that benefit downstream processes by extending shelf life of extracted oils, or by producing cotton in particular colors, for example. Furthermore, recombinant (that is, synthetic) forms of materials such as recombinant bovine somatotropin (rBST), chymosin (rennin), and vaccines are used to increase milk output, facilitate cheese production, and bolster immunity, respectively (20).
The developments arising from agricultural biotechnology, in addition to being profitable, are intended to improve food availability as well as food security. However, these benefits are tempered by concerns for the impact on the environment such as reduction in variation, as well as increased risks to consumers for allergies and malignancies (19). These latter concerns have prompted many countries to adopt strict labeling policies for GMO or GMO-containing products. To address this, biosensor technologies have arisen recently including affinity-based matrix-type assays meant to detect proteins or nucleic acids that are not native to a particular crop (18). These require targeted analysis based on known or suspected entities. The shortcoming of protein-based assays is that the protein may not be produced if the gene is not expressed under the conditions assayed and so the presence of the gene may be missed (13). Within the field of genetic detection, barcode-labeled SERS tags are commonly used, which combine a SERS active metal, typically at the core; a reporter, or barcode, molecule with a unique Raman signature; and a functionalized oligonucleotide layer that targets the genes, encoding proteins of interest. Using multiple reporter molecules, each with a unique Raman signature (barcode), allows for quantitative, multiplexed genetic screening. This type of SERS-barcoded nanosensor was found to be as sensitive as real time PCR when screening genetically modified rice for the Bacillus thuringiensis genes that were introduced to confer insect resistance (21). The detection limit of the Bt gene was 0.1 pg/mL, and the detection limit of the GMO rice mixed in normal rice was 0.1% (w/w).
The main benefits to using SERS-tag-based Raman over traditional fluorescence-tag PCR are the portability and lower cost of SERS systems and a significantly lower detection limit, which eliminates the need for thermal cycling for DNA amplification. However, preparation of test samples for SERS-based analysis is still quite cumbersome. Guven, Uluok, and colleagues (13,22) developed a dual-probe magnetic separation SERS system to optimize sample preparation, requiring only a single hybridization step (13,22). Using this technique, two complementary probes to the gene of interest are functionalized onto two different SERS-active components; either two nanoparticles, or a nanoparticle and substrate. Then, in the single-step hybridization, the targeted genetic material simultaneously binds to both probes, creating a gold-target-gold sandwich. Use of multiple gold layers and nanorod geometry provides a significantly enhanced SERS signal compared to nanospheres, with detection limit concentrations as low as 34 fM, and target concentrations of 10 pM-1 µM.
Monitoring Emissions from Concentrated Animal Feeding Operations
The density of animals in feeding operations and the release of both gaseous and particulate matter has led the Environmental Protection Agency (EPA) to monitor emissions in response to concerns from environmentalists (6). The Natural Resources Conservation Service has made recommendations to producers in an effort to improve air quality that will likely become legislated. However, informative metrics will need to be developed that report on the emission source and apportionment, as well as size distribution of the particulate matter. Some studies have examined exposure time and concentrations of particulate matter based on size and mass, but these have overlooked the source of material being generated. Depending on the source, these emissions may harm both humans and livestock and include methane, ammonia, and microbial contamination in fugitive dust (23). As early as 1929, investigators at the California Institute of Technology were applying Raman to polyatomic gases such as methane, ammonia, and carbon dioxide to determine fundamental discriminating frequencies that would allow for their detection (24). Four decades later, in the early 1970s, Raman was again applied, but this time methods and devices were developed for remote sensing of air pollutants, where air quality could be monitored from a distance of a half mile under varying weather conditions (25). More recently, the technology has been used to directly address the emission concerns surrounding the farming industry. Using a cutoff particle size of 10 µm (PM10), Huang and colleagues (23) generated a Raman spectral library of materials gathered from road dust, pen manure, and animal feed to compare to material gathered on filters of ambient air at a large commercial farm in Kansas over a period of two days. The sensitivity of their assay allowed them to conclude that the majority of the particles (78%) originated from the pen manure, whereas dust from a dirt road (19%) and particles from feed contributed much less to the total collected material. Even upwind of the agricultural feeding operation (AFO), manure was the significant contributor to the particulate matter collected. Another study by Hiranuma and colleagues (26,27) classified particulate into categories of organic, inorganic, and black or brown carbon. Nonagricultural papers have described the specific Raman composition of carbon and other inorganic aerosol particulate matter (28,29). Developing an automated method for rapid screening of individual particles detected during varied weather conditions could predict meteorological and agronomical impacts on dissemination of particulate matter to the surrounding environment. A drawback of using Raman spectroscopy alone is that not all target particles of interest are Raman active. Thus, a complementary imaging modality would be required to positively identify these species. As such, several papers have reported results using a combination of imaging methods, most notably Fourier transform infrared (FT-IR) spectroscopy (28,30), combined with Raman spectroscopy.
While identification of individual particulate matter provides detailed information on the source of aerosol contamination, which is useful to the scientific and research community, more practical systems must be considered for the agriculture industry (31–34). Modern remote sensors in agriculture have evolved, and now include wireless sensor networks that can report on everything from soil and leaf moisture to pH (35,36). These systems could be modified to include Raman sensing wirelessly transmitted to a home monitoring station. The following paragraphs describe three potential remote Raman sensing applications that could be adapted for concentrated animal feeding operation (CAFO) use.
To integrate spectral sensing between airborne and field measurements, drones or unmanned aerial vehicles (UAVs) have been fitted with spectrometers that can detect composition of pesticides as well as field runoff (37). Technology and sensors for UAVs have advanced to include collision avoidance systems and environmental sensing (38,39), and Raman sensing applications have been proposed for a number of years (40–45). Circle Holdings, Inc., was manufacturing the technology for the defense industry in the form of a mini-Raman lidar system (MRLS) (46) that was also licensed to monitor the HVAC systems of large buildings. Despite these developments, commercial use of UAVs is still limited in the United States, where we lag about three years behind Europeans in implementing the technology for daily use (47).
Portable Raman standoff systems have also been developed for detecting small concentrations of aerosol particles at distances ranging from 10 m to 120 m in varying weather and daylight conditions (48,49). Using this method, scattered light is collected through a telescope rather than with a standard microscope lens. If optimized for low-level CAFO aerosol detection, these systems could be adapted for remote sensing of airborne agricultural byproducts.
Alternately, Piorek and colleagues (50) described a microfluidic device that can adsorb and aggregate water-soluble airborne particles to silver nanoparticles. SERS is then used to image the varying aggregates through the length of the sensor. Modifications to this design could also be developed to monitor various constituents of interest in agriculture.
Water Monitoring and “Numeric Nutrient Criteria”
The leaching of fertilizer runoff from agricultural lands into tributaries has had repercussions on the viability of coastal waters, such that a number of studies on controlling drainage and managing runoff have been performed (7,51,52). For marine life, the critical concentration of oxygen in water is approximately 4 mg/L. As a result of agricultural runoff, nitrogen and phosphorus from fertilizers have led to hypoxic (oxygen under 2 mg/L water) and anoxic (0 mg/L) dead zones in the Gulf of Mexico and the Chesapeake Bay. Coastal dead zones come about as oxygen is depleted by microbial feeding and degradation of algal blooms that are brought on by eutrophication, where the concentrated nutrient discharge from agricultural runoff leads to algal overgrowth (53). It was reported in 2013 that the actual dissolved oxygen content is unknown for 75% of global dead zones (54). However, as atmospheric nitrogen saturates seawater and generally remains constant, it can be used as a reference point against which fluctuations in the ratio of nitrogen to dissolved oxygen serves as an indicator of changes in dissolved oxygen. This ratio has been determined to be 1.78 in seawater, based on the assumption that there is 100% saturation of both gases at 1 atm. Water does not scatter Raman spectra as it does those spectra generated by FT-IR, so Raman is an ideal probe to monitor aqueous environments. Currently, ship-based optodes are used to make in situ measurements as a vessel traverses the area to be measured (54). However, because of travel time and distance there is a natural variation in water conditions that prevents temporal standardization. An option is in development now that pulses an ultraviolet (UV) laser into water from an aircraft to measure the Raman emission that is scattered back to a lidar detector (54). To measure the nitrogen to oxygen ratios, their Raman signals (at 387.55 nm and 376.2 nm, respectively) collected from water were compared with Raman signals in air, and concentration was determined based on calibration curves accounting for water temperature and gas saturation. Using this technique, calibration curves ratioed to the water bending peak have enabled quantitative measurement of oxygen and nitrogen in water with accuracy that approaches in situ measurements. While the technique does require a significant number of acquisitions and high spectral resolution to achieve the signal of dissolved gas in water, the use of drones and lidar technology allow the process to be automated.
Submersible systems for Raman measurement have also been developed, such as the deep-ocean Raman in situ spectrometer (DORISS) (55–58). Commercial spectrometers can be repackaged into three individual components (spectrometer, laser-power supply telemetry, and optical head) in such a way so as to withstand extreme pressure and temperature conditions. Measurements of gas, liquid, and solid materials have been performed at conditions as extreme as 3600 m underwater at 1.6 °C.
For detailed point-sampling of water specimens, a number of techniques have been examined. Gajaraj and colleagues (59) used SERS to identify nitrate in wastewater at concentrations of 1–100 mg NO3/L (1–100 ppm), and at even broader concentrations in pure water using a commercial gold nanosubstrate. Although the presence of phosphates can confound nitrate measurement, results are comparable with other chromatography methods. Resonance Raman spectroscopy uses excitation wavelengths that are within the absorption bands of the target analytes to selectively enhance their Raman response. Ianoul and colleagues (60) measured dissolved nitrite and nitrate at concentrations of 14 µM (<200 ppb) in water, and were also able to detect NO2- in treated wastewater sludge traced to loss of Nitrobacter from the treatment process.
The ability to monitor water and liquids directly without transfer to separate vessel or the need for chemical tag has been achieved by marrying acoustic levitation and Raman sampling, with some corrective considerations (61,62). Using these techniques, Wood and colleages (63) were able to examine algal samples for chlorophyll a (chlA) and β-carotene content in nitrogen-rich and nitrogen-poor conditions, and taxonomic determinations were made based on the ratios of these two components. The effects of moisture loss during levitation and exposure to white light were also examined. Under nitrogen-limited conditions, the amount of chlA was found to be markedly reduced. Because the chromophores form a lattice that is the sum of excitonic coupling states, the Raman spectra were the result of aggregated states and could be used to determine the relative amounts of functional chlA in algal and environment-specific readouts. Numerous agricultural applications would be amenable to such analyses.
Future Developments and Conclusions
While many of the studies mentioned were developed specifically for agricultural and environmental applications, others were developed for defense or other industries. Transitioning a system to agricultural applications may be as simple as developing a database and accompanying algorithms based on the analytes of interest, or it may involve developing new SERS particles, hardware packaging, and remote communication capability.
In early studies of agricultural and environmental applications of Raman spectroscopy, there were a number of barriers that were preventing it from being a truly viable commercial tool, including spectral resolution, Raman microscope size, and measurement time. In the past decade, a number of portable Raman systems have become commercially available with spectral resolution as low as 6 cm-1, making Raman commercially viable for some agricultural applications. However, many of the applications described here will require a tool with significantly higher resolution and more sensitive detectors at a cost that is feasible for the average farm or inspector. Power supply and cooling issues must also be addressed for some portable applications. Sample preparation and focusing techniques must be integrated into the sensor to streamline measurements and focusing, and data acquisition and analysis must be automated. As light sources, charge-coupled devices, and avalanche photodiode technologies become more affordable and components become miniaturized for portability (64), the full potential of Raman spectroscopy for agricultural monitoring will be realized.
References:
(1) R. Thomas and K.A. Bakeev, Raman Technology for Today’s Spectroscopists supplement to Spectroscopy29(s6), 34–41 (2014).
(2) H.G.M. Edwards and T. Munshi, Anal. Bioanal. Chem.382(6), 1398–1406 (2005).
(3) R.E. Kast, S.C. Tucker, K. Killian, M. Trexler, K.V. Honn, and G.W. Auner, Cancer Metastasis Rev.33(2–3), 673–693 (2014).
(4) S.K. Freeman, J. Agr. Food Chem.21(4), 521–525 (1973).
(5) D.T. Yang and Y.B. Ying, Appl. Spectrosc. Rev.46(7), 539–560 (2011).
(6) T. Janzen, “Modern Agriculture’s Big Five Issues (and How the Law is Reacting to Them)” in LexisNexis Legal Newsroom Environmental (2013). Available at: http://www.lexisnexis.com/legalnewsroom/environmental/b/publichealthsafety/archive/2013/10/15/modern-agriculture-39-s-big-five-issues-and-how-the-law-is-reacting-to-them.aspx.
(7) U.S. Environmental Protection Agency. State Development of Numeric Criteria. Office of Water, Office of Science and Technology, Washington DC. (Last accessed August 11, 2015) Available at: http://cfpub.epa.gov/wqsits/nnc-development/.
(8) Y.H.P. Hsieh and J.A. Ofori, J. Agr. Food Chem.62(52), 12536–12544 (2014).
(9) K.I. Ivanov, K. Eskelin, A. Lohmus, and K. Makinen, J. Gen. Virol.95, 1415–1429 (2014).
(10) J.L. Jacquet and D. Pauly, Mar. Policy32(3), 309–318 (2008).
(11) R. Jacquet, C. Miege, P. Bados, S. Schiavone, and M. Coquery, Environ. Toxicol. Chem.31(2), 279–288 (2012).
(12) N.D.T. Uyen, “Asian Seafood Raised on Pig Feces Approved for U.S. Consumers” in Bloomburg Business (2012). Available at: http://www.bloomberg.com/news/articles/2012-10-11/asian-seafood-raised-on-pig-feces-approved-for-u-s-consumers.
(13) B. Guven, I.H. Boyaci, U. Tamer, and P. Calik, Analyst137(1), 202–208 (2012).
(14) M. Sourdaine, A. Guckian, C. Harvey, D. Guenther, and Y. Mattley, Raman Technology for Today’s Spectroscopists supplement to Spectroscopy29(s6), 24–32 (2014).
(15) C. Eliasson, N.A. Macleod, and P. Matousek, Anal. Chim. Acta607(1), 50–53 (2008).
(16) S.M. Watkins, B.D. Hammock, J.W. Newman, and J.B. German, Am. J. Clin. Nutr.74(3), 283–286 (2001).
(17) A.M. Herrero, Food Chem.107(4), 1642–1651 (2008).
(18) M.A. Arugula, Y.Y. Zhang, and A.L. Simonian, Anal. Chem.86(1), 119–129 (2014).
(19) “Pros and Cons of Genetically Modified Foods,” on HealthResearchFunding.org (2013). Available at: http://healthresearchfunding.org/pros-cons-genetically-modified-foods/.
(20) Modern biotechnology and agricultural markets: A discussion of selected issues. A publication of the Directorate for Food, Agriculture and Fisheries Committee for Agriculture, from the Working Party on Agricultural Policies and Markets, under the Organisation for Economic Co-operation and Development. AGR/CA/APM(2000)5/FINAL. Available at: http://www.iatp.org/files/Modern_Biotechnology_and_Agricultural_Markets_.htm (Last accessed August 11, 2015).
(21) K. Chen, H.Y. Han, Z.H. Luo, Y.J. Wang, and X.P. Wang, Biosens. Bioelectron.34(1), 118–124 (2012).
(22) S. Uluok, B. Guven, H. Eksi, Z. Ustundag, U. Tamer, and I. Boyaci, J. Nanopart. Res.17(1), 1–12 (2015).
(23) Q. Huang et al., Atmos. Environ.66, 17–24 (2013).
(24) R.G. Dickinson, R.T. Dillon, and F. Rasetti, Phys. Rev.34(4), 0582–0589 (1929).
(25) T. Hirschfeld, E.R. Schildkraut, H. Tannenbaum, and D. Tanenbaum, Appl. Phys. Lett.22(1), 38–40 (1973).
(26) Y. Dellaa, C. Rahmoune, J. Kister, and N. Dupuy, Aip Conf. Proc.1267, 529–530 (2010).
(27) N. Hiranuma, S.D. Brooks, J. Gramann, and B.W. Auvermann, Atmos. Chem. Phys.11(16), 8809–8823 (2011).
(28) J.S. Gaffney, N.A. Marley, and K.J. Smith, J. Phys. Chem. A119(19), 4524–4532 (2015).
(29) N.P. Ivleva, U. McKeon, R. Niessner, and U. Poschl, Aerosol Sci. Tech.41(7), 655–671 (2007).
(30) H.J. Jung, H.J. Eom, H.W. Kang, M. Moreau, S. Sobanska, and C.U. Ro, Analyst139(16), 3949–3960 (2014).
(31) S.K. Sharma, P.G. Lucey, M. Ghosh, H.W. Hubble, and K.A. Horton, Spectrochim. Acta,Part A59(10), 2391–2407 (2003).
(32) E.C. Cull, M.E. Gehm, B.D. Guenther, and D.J. Brady, Proc. SPIE, Chemical and Biological Sensors for Industrial and Environmental Security,5994(0H), 59940–59941 (2005).
(33) E.C. Cull, M.E. Gehm, S.T. McCain, B.D. Guenther, and D.J. Brady, Proc. SPIE, Sensors, and Command, Control, Communications, and Intelligence (C3I) Technologies for Homeland Security and Homeland Defense IV, 5778, 376–382 (2005).
(34) C.E. Corrigan, G.C. Roberts, M.V. Ramana, D. Kim, and V. Ramanathan, Atmos. Chem. Phys.8(3), 737–747 (2008).
(35) N. Sakthipriya, Middle-East Journal of Scientific Research20(9), 1127–1132 (2014).
(36) P. Sun, Proceedings - 4th International Conference on Intelligent Computation Technology and Automation, ICICTA 20112, 189–193 (2011).
(37) R. Morris, “Aerial spectroscopy for crop monitoring” in Ocean Optics News and Events Archives (November 2014). Available at http://oceanoptics.com/aerial-spectroscopy-crop-monitoring/ (Last accessed August 11, 2015).
(38) R.K. Mehra, J. Byrne, and J. Boškovic, AUVSI’s Unmanned Systems North America 2005 - Proceedings 985–999 (2005).
(39) R.J. Black and B.M. Moslehi, Proc. SPIE,7817, 78170L (2010).
(40) A.J. Sedlacek III, M.D. Ray, N.S. Higdon, and D.A. Richter, Proc. SPIE4577, 95–104 (2002).
(41) M.D. Ray and A.J. Sedlacek III, Proc. SPIE3707, 138-47 (1999).
(42) A. Malinka, in Light Scattering Reviews 2 A. Kokhanovsky, Ed. (Springer Berlin Heidelberg, 2007), pp. 125–155.
(43) D.N. Whiteman, G. Schwemmer, K. Rush, I. Veselovskii, B. Demoz, Z. Wang, W. Welch, L. Ramos, and R. Rallison, ”Raman airborne spectroscopic lidar (RASL) - final report. 2002. Available at: http://ramanlidar.gsfc.nasa.gov/instruments/raman%20airborne%20spectroscopic%20lidar/rasl-final-report.pdf (Last accessed August 11, 2015).
(44) D.N. Whiteman, B. Demoz, P. Di Girolamo, J. Comer, I. Veselovskii, K. Evans, Z. Wang, M. Cadirola, K. Rush, G. Schwemmer, B. Gentry, S.H. Melfi, B. Mielke, D. Venable, and T. Van Hove, J. Atmos. Oceanic Technol.23, 157–169 (2006).
(45) Lidar remote sensing for environmental monitoring xiv. Lidar Remote Sensing for Environmental Monitoring XIV, 13 Oct 2014. USA: SPIE; 2014. Available at: http://proceedings.spiedigitallibrary.org/volume.aspx?volumeid=16792 (Last accessed August 11, 2015).
(46) S. Cohen, “Circle Group Holdings, Inc. announces its plan to develop the mini Raman Lidar System for unmanned aerial vehicle applications in the defense industry” in PR Newswire (2005) Available at http://www.prnewswire.com/news-releases/circle-group-holdings-inc-announces-its-plan-to-develop-the-mini-raman-lidar-system-for-unmanned-aerial-vehicle-applications-in-the-defense-industry-54421617.html. (Last accessed August 11, 2015).
(47) C. Garling, “Drone, Drone on the Range” in Modern Farmer (2013). Available at: http://modernfarmer.com/2013/07/drones-drones-on-the-range/.
(48) J.C. Carter, S.M. Angel, M. Lawrence-Snyder, J. Scaffidi, R.E. Whipple, and J.G. Reynolds, Appl. Spectrosc.59(6), 769-775 (2005).
(49) A. Pettersson, I. Johansson, S. Wallin, M. Nordberg, and H. Ostmark, Propell. Explos. Pyrot.34(4), 297–306 (2009).
(50) B.D. Piorek, S.J. Lee, J.G. Santiago, M. Moskovits, S. Banerjee, and C.D. Meinhart, P. Natl. Acad. Sci. USA104(48), 18898–18901 (2007).
(51) M. Fink, P. Varghese, J. Borysow, W. Gardner, and F. Shafiei, “Compact Raman Spectrometer for Analysis of The Nitrification in The Gulf of Mexico Due to Extensive Nutrient Loading,” in Optics InfoBase Conference Papers, Optical Instrumentation for Energy and Environmental Applications, E2 2011, Austin, Texas, November 2–3, 2011.
(52) “Farm Run-Off: Down the Mississippi to the Gulf of Mexico,” in North Carolina State University Results 9(2) 2007. Available at: http://www.ncsu.edu/research/results/vol9n2/07.html. (Last accessed August 11, 2015).
(53) D. Biello, “Oceanic Dead Zones Continue to Spread,” in Scientific American (2008). Available at: http://www.scientificamerican.com/article/oceanic-dead-zones-spread/. (Last accessed August 11, 2015).
(54) R. Ganoe and R.J. DeYoung, “Remote Sensing of Dissolved Oxygen and Nitrogen in Water Using RamanSpectroscopy,” in NASA Technical Memorandum 2013–218142 (2013). Available at: http://permanent.access.gpo.gov/gpo50478/20140005468.pdf. (Last accessed August 11, 2015).
(55) W.J. Kirkwood, S.N. White, M. Brown, R. Henthorn, S. Jensen, K.A. Salamy, E.T. Peltzer, and P. Brewer, in Oceans 2003. Celebrating the Past ... Teaming Toward the Future (IEEE Cat. No.03CH37492) 2, 838–43 (2003).
(56) J.D. Pasteris, B. Wopenka, J.J. Freeman, P.G. Brewer, S.N. White, E.T. Peltzer, and G.E. Malby, Appl. Spectrosc.58(7), 195A–208A (2004).
(57) S.N. White, W. Kirkwood, A. Sherman, M. Brown, R. Henthorn, K.A. Salamy, E.T. Peltzer, P. Walz, and P.G. Brewer, “Laser Raman Spectroscopic Instrumentation for In Situ Geochemical Analyses in the Deep Ocean,” presented at Oceans 2004 Marine Technology Society (MTS)/ Institute of Electrical and Electronics Engineers (IEEE) Techno-Ocean 2004, Piscataway, New Jersey, 2004.
(58) P.G. Brewer, G. Malby, J.D. Pasteris, S.N. White, E.T. Peltzer, B. Wopenka, J. Freeman, and M.O. Brown, Deep Sea Research Part I: Oceanographic Research Papers51(5), 739–753 (2004).
(59) S. Gajaraj, C. Fan, M.S. Lin, and Z.Q. Hu, Environ. Monit. Assess.185(7), 5673–5681 (2013).
(60) A. Ianoul, T. Coleman, and S.A. Asher, Anal. Chem. 74(6), 1458–1461 (2002).
(61) J. Schenk, U. Panne, and M. Albrecht, J. Phys. Chem. B116(48), 14171–14177 (2012).
(62) R. Tuckermann, L. Puskar, M. Zavabeti, R. Sekine, and D. McNaughton, Chemical Analysis of Acoustically Levitated Drops by Raman Spectroscopy, 5th Ed. (Springer Verlag, 2009)pp. 1433–1441.
(63) B.R. Wood, P. Heraud, S. Stojkovic, D. Morrison, J. Beardall, and D. McNaughton, Anal. Chem.77(15), 4955–4961 (2005).
(64) J. Malinen, A. Rissanen, H. Saari, P. Karioja, M. Karppinen, T. Aalto, and K. Tukkiniemi, Proc. SPIE9101, (2014) doi:10.1117/12.2053567.
Stephanie C. Tucker and Kenneth V. Honn are with the Bioactive Lipids Research Program in the Department of Pathology at Wayne State University in Detroit, Michigan. Rachel E. Kast and Gregory W. Auner are with the Michael and Marian Ilitch Department of Surgery at Wayne State University and Biomedical Engineering, Smart Sensors and Integrated Microsystems in Detroit, Michigan.
Direct correspondence to: stucker@med.wayne.edu

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.