Application of SERS to the Determination of Butylated Hydroxyanisole in Edible and Essential Oils
Spectroscopy
Surface-enhanced Raman scattering (SERS) has been applied to the determination of the antioxidant butylated hydroxyanisole (BHA), commonly used in fatty foods and oils to prevent their oxidation. The use of SERS-Raman microscopy with an inexpensive homemade silver substrate allowed the direct determination of BHA in oils without any sample handling. Several edible and essential oils (used as flavorings) have been considered for this purpose.
Surface-enhanced Raman scattering (SERS) has been applied to the determination of the antioxidant butylated hydroxyanisole (BHA), commonly used in fatty foods and oils to prevent their oxidation. The use of SERS Raman microscopy with an inexpensive homemade silver substrate allowed the direct determination of BHA in oils without any sample handling. Several edible and essential oils (used as flavorings) have been considered for this purpose. The experimental conditions, the peculiarities of each type of oil, and the characteristics of silver substrate, including scanning electron microscopy (SEM) analysis, are shown and discussed.
Lipid oxidation is catalyzed by high temperature and light. The result of this process is the production of volatile aldehydes and ketones responsible for lipid off-flavors and off-odors (1). Oxidation of lipids is undesirable from the point of view of the food industry as consumption of rancid fats is hazardous to health as well as unpleasant. Toxic substances can be produced and the nutrient value of fats is lost during the rancidity process (2). This phenomenon can be prevented by the addition of antioxidants.
Butylated hydroxyanisole (BHA, designed as “E 320” in the Annex II of Regulation (EC) No 1333/2008) is a synthetic antioxidant commonly used as a preservative of fats, fat additives such as essential oils, fat containing products, and vitamins. This phenolic antioxidant consists of a mixture of 4-methoxy-2-tertbutyl phenol (2-BHA) and 4-methoxy-3-tertbutyl phenol (3-BHA) (3) and is listed as a food additive (E 320). It has been demonstrated to have singlet oxygen quenching capabilities. Singlet oxygen is extremely reactive to lipids causing their quick deterioration (4). Fernandez-Alvarez and colleagues (5) investigated the antioxidant capacity of BHA as well as its combination with other natural and synthetic antioxidants. The antioxidant activity of a 0.2 mM solution of BHA (95% ethanol-5% water) with a slightly modified 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay was determined as 0.36 ± 0.04 mM of Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) equivalents.
All food additives must comply with European legislation that regulates the list of authorized substances and their approved conditions of use (6). The maximum allowed level of BHA added to edible oils is established as 200 μg/g (expressed on fat) and 1000 μg/g when added to essential oils.
Several chromatographic methods for BHA determination have been widely described in literature. Saad and colleagues (7) proposed a method using a reversed-phase high performance liquid chromatography (HPLC). In this case, sample pre-treatment was applied using a methanol-acetonitrile extraction of BHA in cooking oils. Xu and colleagues (8) also developed a HPLC method with detection based on chemiluminescence reaction. On the other hand, Yang and colleagues (9) published work about a rapid gas chromatography (GC) method for direct determination of BHA.
In the case of Raman spectroscopy, the surface-enhanced Raman scattering (SERS) method for the detection of BHA was developed and evaluated by Yao and colleagues (10). These authors proposed gold SERS-active substrate for quantification of BHA in methanol, although no real food matrix was used.
The aim of this work was to develop a new method for BHA determination in different types of both edible and essential oils by Raman microscopy, without the need for any sample handling or pretreatment. Obviously, oil is one of the most difficult matrices to analyze because of its viscosity and complexity of composition. A novel solution for this problem was applied in this work. The Raman signal derived from oil was decreased by lowering the temperature of the measurement, and simultaneously the analyte signal was enhanced using silver nanoparticles as a SERS substrate. In this regard, an inexpensive homemade substrate has been prepared by a tartrate-based reduction of a silver solution. The deposition of a metallic silver layer on glass to obtain a mirror is applied commonly nowadays and has been used for many years. For instance, a description was given by J. von Liebig (11) in 1868, or by W. Simon in 1884 (12,13). In this work, the preparation was optimized based on the original recipes.
The proposed method is quick and does not require any solvents during the sample preparation. It has been conceived as a screening method for edible and essential oil samples fortified with BHA. Surely, this method can be applied as a good complement to standard chromatographic analysis. Moreover, the developed procedure can determine the effectiveness of BHA prevention after thermal oil degradation.
Materials and Methods Chemicals
Butylated hydroxyanisole (≥98.5%), silver nitrate (≥99.0%), and nitric acid (70%, ACS reagent) were obtained from Sigma-Aldrich, ethanol absolute and methanol were obtained from Panreac, ammonium hydroxide solution (32%) was obtained from Sharlau, and potassium sodium tartrate tetrahydrate (≥99%) was obtained from Merck Millipore.
The essential oils from basil (Ocimum basillicum), cinnamon leaves (Cinnamomum zeylanicum), citronella grass (Cymbopogon winterianus), cumin seeds (Cuminum cyminum), dill seeds (Anethum graveolens), ginger (Zingiber officinale), clove (Syzygium aromaticum), oregano (Origanum vulgaris), rosemary (Rosmarinusofficinalis), and thyme (Thymus vulgaris) were supplied by Argolide.
The commercial sunflower oil and olive oil were bought in a local supermarket. Edible oils were stored in a dark place at room temperature.
Ultrapure water (18 MΩ·cm) was obtained from a Millipore Milli-Q PLUS 185 system. Nitrogen (99.999%) was provided by Air Liquide.
Equipment
A Thermo Scientific DXR Raman spectrometer equipped with a microscope, an excitation laser source at 532 nm, and a motorized microscope stage sample holder was used. A charge-coupled device (CCD) detector was used for SERS measurements.
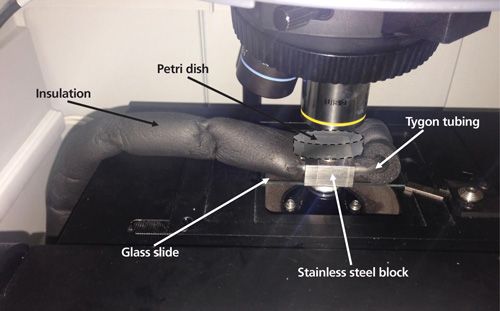
For -2 °C measurements, a homemade cooling system was developed with a Thermo-Haake K20 refrigerated bath with a C10 immersion circulator. Tygon tubing covered with air-conditioning pipe insulation was connected to the external circuit to cool a stainless steel block with two 8-mm orifices. The block was stuck to a standard glass slide and placed into the microscope stage. Petri dishes containing the samples were put on top of the block. Figure 1 shows an image of the system.
A scanning electron microscopy (SEM) image of silver substrate was obtained with a FEI Inspect F system at Nano Facility Piemonte, Istituto Nazionale di Ricerca Metrologica (INRIM).
Preparation of Samples
The samples of olive oil and sunflower oil were prepared by fortifying them with 200 μg/g of BHA, whereas essential oils from basil, cinnamon, oregano, and rosemary were fortified at 1000 μg/g with BHA. All the samples were heated at 40 °C to facilitate the dissolution of BHA. Subsequently, they were mixed with a vortex shaker from Velp Scientifica. All of the samples were stored in the dark at room temperature.
BHA Degradation
The samples of 200 μg/g of BHA in sunflower oil and pure sunflower oil were thermally degraded at 180 °C for three time periods: 5, 10, and 15 min. Three replicates of each sample were prepared.
SERS Substrate
The well known reaction of silver mirror (11-13) was applied to obtain a silver layer on a glass Petri dish. Different concentrations, reaction times, and temperatures were tested. The following corresponds to the optimized recipe. First, 15 mL of 6.2% (w/v) aqueous solution of silver nitrate was prepared. A small portion (about 1 mL) was reserved. Diluted ammonia solution was added drop by trop until the chocolate-colored precipitate was just dissolved. Then the remaining 1 mL of silver nitrate solution was added, showing an incipient turbidity. The solution was diluted up to 100 mL with ultrapure water, and then it was kept in a brown glass bottle.
Second, the reducing solution was prepared as follows: 0.19 g of potassium sodium tartrate was added to 100 mL of boiling water. Then 20 mL of 1.1% (w/v) aqueous silver nitrate solution was slowly added with strong stirring. The solution was left to boil for 10 min. After it was air-cooled to room temperature, the obtained solution was filtered with a cellulose acetate syringe filter (membrane diameter: 25 mm, pore size: 0.50 μm) and kept in a brown glass bottle. It is necessary to prepare the solutions monthly. The freshness of the solutions was checked by examining the initial reaction time. The deposition of silver nanoparticles should start approximately 20 s after the solutions are mixed.
Soda-lime glass Petri dishes of 60 mm diameter and 15 mm height from Brand were washed with ethanol and dried under a strong stream of nitrogen. Then, 2 mL of the silver solution and 2 mL of the reducing solution were added and mixed to obtain a silver mirror consisting of silver nanoparticles deposited on the glass surface. Figure 2 shows an SEM image (120,000×) of the SERS silver substrate. As can be seen, prismatic nanoparticles with sizes of about 80-230 nm were obtained.

Measurement Conditions
Before data collection the Raman microscope was aligned and calibrated. The Raman system we used has an automatic alignment and calibration procedure to ensure and maintain optimal performance. The aligning process allows the three beams (laser excitation, visible light, and Raman scattering) to be aligned on the same optical path, and calibration (carried out by using neon and polystyrene standards) ensures a correct interpretation of the data (intensity and peaks frequency). The alignment and calibration were done using the tool provided with the system. In the case of SERS measurement, the laser was focused with a 10× objective (0.25 NA) to get a spot of about 2 μm. Aperture was set to a 50-μm pinhole, the grating was 900 lines/mm, and the spectral range was 3500-50 cm-1. All of the spectra were acquired using a laser power of 0.5 mW (measured at the sample) and an exposure time of 30 s for 20 exposures. Fluorescence correction and cosmic ray rejection were applied. Moreover, a background was used to characterize the dark noise of the CCD to improve data quality and signal-to-noise ratio (S/N). The collection and analysis of all the spectra were performed using the software Omnic v. 9.2 from Thermo Fisher Scientific.
Analysis of Samples
Solutions of BHA in the following oils were analyzed: olive oil, sunflower oil, basil essential oil, cinnamon essential oil, oregano essential oil, and rosemary essential oil. First, 11 mL of the sample was deposited on the silver-coated Petri dish. The measurement was done at -2 °C. Three spectra of each replicate were collected to check repeatability of the Petri dish, and three replicates of each sample were prepared to check reproducibility of silver substrate. Also, the samples deposited on the glass Petri dish without silver substrate were analyzed to obtain the enhancement factor.
Results and DiscussionOptimization of Measurement Conditions
A total of 10 pure essential oils were tested to obtain their Raman spectra. Each sample was placed in a 2-mL glass vial and its spectrum was measured with a laser power of 8 mW at the sample and an exposure time of 10 s for four exposures. Following this preliminary testing we chose four (basil, cinnamon, oregano, and rosemary) out of the 10 oils because of their low fluorescence (further analysis with different laser excitation are ongoing and will be presented in another publication). Regarding the oil sample size, three amounts of sample deposited on the Petri dish were tested: 1 drop, 11 g, and 22 g. The optimum amount demonstrated was 11 g.
Finally, the conditions of measurement were optimized to obtain the highest SERS signal, maximize S/N, and avoid CCD overflow.
Assessment of SERS Effect, Characteristic BHA Peaks, and Influence of Measurement Temperature
In a preliminary study in our laboratory (unpublished results), the SERS effect of our synthesized silver substrate was tested using melamine. This molecule is frequently used in SERS studies with gold (14) or silver (15) substrates because of its good performance. In our case, an enhancement factor higher than 115 was obtained. A drop of a 10-mg/L aqueous solution was placed on the silver surface and carefully evaporated under a nitrogen stream. The next step was to prepare a methanolic solution of BHA in a similar way as the work of Yao and colleagues (10) with gold colloidal nanoparticles. Despite the fact that SERS was noticed, the acquired spectrum was quite different, thus indicating that differences in the substrate (colloidal gold versus rigid silver substrate) led to different results.
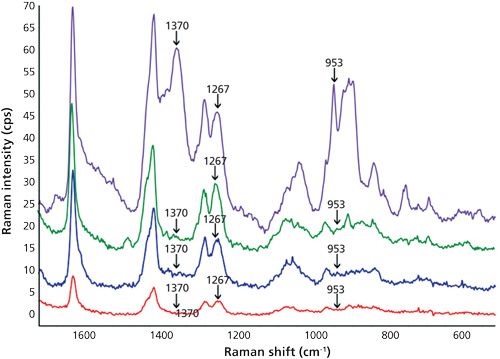
Then, the assignment of the characteristic peaks of BHA in oil was carried out. As shown in Figure 3, Raman peak shifts at 1370 cm-1, 1377 cm-1, 1267 cm-1-probably because of in-plane ν(CH) swing according to Yao and colleagues (10)-and 953 cm-1 were unequivocally caused by BHA. A significant drop in the intensities of sunflower oil bands at 1047 and 684 cm-1 because of the addition of BHA was also observed. The two lower traces of Figure 3 clearly demonstrate a significant increase in the signal as a consequence of SERS in comparison with normal Raman.
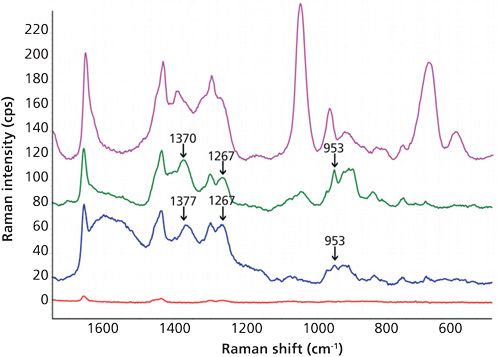
As a novelty, in this work low sample temperature (-2 °C) during SERS measurement was tested and the results were compared to those obtained at room temperature (about 25 °C). Despite the results obtained by other authors who have explored the influence of temperature over SERS effect (16,17), we did not observe an obvious pattern or behavior since other influencing variables such as pH were jointly considered. Figures 3 and 4 clearly show that better definition and sensitivity were obtained when lowering the temperature. In particular, the sharper bands of the cold pure oil in the region 1580-1650 cm-1 and the 1190 cm-1 peak widening at room temperature which disappeared at -2 °C were indicative of changes in oil vibrations because of the addition of BHA. This effect was not noticed in methanol because of its simple chemical composition, whereas oil is a very complex matrix. As a proposal for a future study, the influence of the addition of different antioxidants into behavior of oil peaks using SERS is considered. A decrease in the signal of the matrix, both edible and essential oils, and an increase in the signals of BHA were noticed when lowering the temperature. In the case of the essential oil of basil, the characteristic BHA peaks were 953 and 897 cm-1, this later peak coincides with an out-of-plane ν(CH) swing (10). Enhancement factors at 897 cm-1 calculated as height ratio between the SERS and the conventional Raman spectra were 23±4 and 34±5 (n = 9%, 95%) for room temperature and -2 °C, respectively. Precision can be considered as satisfactory, taking into account that one of the main SERS drawbacks is the lack of reproducibility caused by the variability of the particle size and morphology of the enhancing substrates (18).
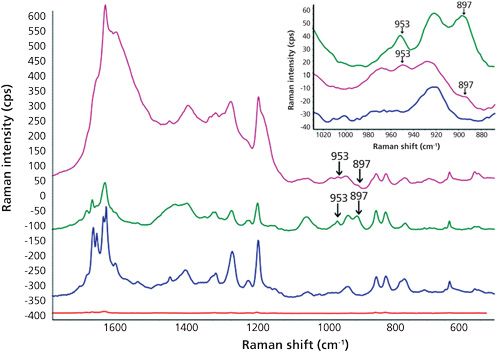
Regarding the enhancement factors, values of 106 or even higher are frequently reported in the literature (19). Nevertheless, the results obtained here are promising because of two reasons: firstly, high enhancement factors are usually achieved by using expensive metal substrates, whereas a cheap, easily homemade silver surface has been used here. Secondly, the deposition of solid analytes on substrates from their aqueous or organic solutions evaporated to dryness is a habitual practice for complete removal of matrix effects. In contrast, BHA has been measured in this study in real liquid oils without any manipulation. Because oils are typically considered a complex matrix, the direct BHA determination constituted a challenge.
SERS Performance at Low BHA Concentration
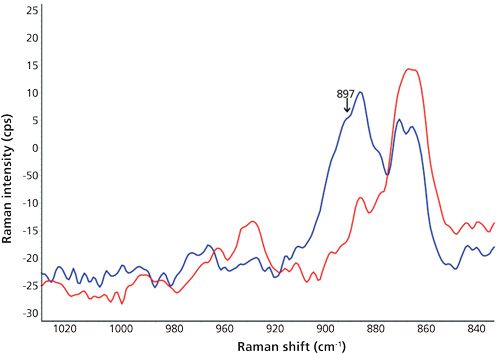
The spectra corresponding to the samples of olive, cinnamon, and rosemary oils fortified with 5% BHA were indistinguishable from the pure oils in all of the cases. This effect was especially notorious in the cinnamon oil, where the intense SERS peaks of cinnamaldehyde completely hid the possible BHA bands. On the other hand, the main goal of this study was reached in the case of oregano oil. Here, the BHA peak at 897 cm-1 of an oil sample fortified at 1000 μg/g (the highest allowed by law) was significantly different from the pure oil, as seen in Figure 5. This means that the proposed method could be successfully applied to the routine control of oregano oil additivated with the maximum legal concentration of BHA without needing any sample preparation.
Control of Oil Samples Subjected to Thermal Degradation
Finally, the thermal stability of BHA in sunflower oil was measured as a function of time. Sunflower oil samples fortified with 5% BHA were heated for 5, 10, and 15 min at 180 °C, a common temperature for fryers. As can be seen in Figure 6, the analysis of the obtained spectra showed a progressive disappearance of the characteristic peaks of BHA over time. After a 5-min degradation time, the Raman shift at 1267 cm-1 was significantly decreased. Moreover, a complete degradation of peaks at 953 cm-1 and 1377 cm-1 was observed just after 5 min. The different decrease in the intensity of bands could be caused by the complex reaction mechanisms of the thermal decomposition of BHA (20). Several reactions are involved including oxidation, etherification, and dimerization, resulting in three possible intermediate compounds and up to six different final products. These substances could have a low or zero interaction with the SERS substrate, or their concentrations could be too low to be detected. Despite the fact that this article is not focused on oil degradation, a decrease of several characteristic shifts of sunflower oil was observed too after 10 min because of its own thermal degradation. In this sense, the behavior of bands in the regions about 1730-1720 cm-1 (>C=O stretch in unsaturated esters) and 1340-1185 cm-1 (>CH=CH<, CH deformation vibration cis and trans) would be interesting in relationship with thermal stress. Unfortunately, no significant differences among spectra were found. Anyway, the versatility of the developed method and its application to the determination of thermal decomposition of BHA has been demonstrated.
Conclusions
The determination of BHA in sunflower, basil, and oregano oil has been successfully accomplished for the first time by a SERS procedure based on an inexpensive homemade silver substrate. The characteristic peak shifts of BHA in oils have been determined. A cooling setup has been developed, and an improved SERS signal has been obtained at a low measurement temperature. The maximum permitted concentration of BHA in oregano essential oil used as a food additive has been detected without any sample handling. Besides, thermal stability of BHA has been checked in sunflower oil. Future work will be focused on improving SERS substrates to decrease the detection limits. This would allow a use of this technique for routine control or for screening purposes.
Acknowledgment
Warm and sincere thanks are given to Thermo Fisher Scientific for the fruitful collaboration and effective exchange of experience and knowledge. Also huge thanks are given to the Thermodynamic Division from Istituto Nazionale di Ricerca Metrologica in Turin, Italy, for providing SEM images of the SERS substrate.
M. Wrona acknowledges the FPU grant (reference number AP2012-2716) received from the MEC, Ministerio de Educación, Cultura y Deporte, Spain.
This work has been financed by the project AGL2012-37886 from the Spanish Ministry of Science and Innovation.
References
(1) B. Matthäus, in Oxidation in Foods and Beverages and Antioxidant Applications, vol. 2, E.A. Decker, D.J. McClements, and D.J. Elias, Eds. (Woodhead Publishing, Cambridge, UK, 2010), pp. 183-238.
(2) C. Dobarganes and G. Marquez-Ruiz, Curr. Opin. Clin. Nutr. Metab. Care.6, 157-163 (2003).
(3) J. Pokorny, N. Yanishlieva, and M. Gordon, Eds. Antioxidants in Food. Practical Applications (Woodhead Publishing, Cambridge, UK, 2001).
(4) J.H. Lee and M.Y. Jung, J. Food Sci.75, C506-C513 (2010).
(5) L. Fernandez-Alvarez, P. del Valle, D. de Arriaga, M.R. Garcia-Armesto, and J. Rua, Food Control42, 303-309 (2014).
(6) Commission Regulation (EU) No 1130/2011. OJ L 295,12.11.2011, pp. 178-204.
(7) B. Saada, Y.Y. Sing, M.A. Nawi, N.H. Hashim, A.S.M. Ali, M.I. Saleh, S.F. Sulaiman, K.M. Talib, and K. Ahmad, Food Chem.105, 1-6 (2007).
(8) S. Xu, F. Chen, M. Deng, and Y. Sui. Luminescence29, 1027-1032 (2014).
(9) M.H. Yang, H.J. Lin, and Y.M. Choong, Food Res. Int.35, 627-633 (2002).
(10) W. Yao, Y. Sun, Y. Xie, S. Wang, L. Ji, H. Wang, and H. Qian, Eur. Food Res. Technol.233, 835-840 (2011).
(11) J. von Liebig. Philos. Mag. 4(35), 146-147 (1868).
(12) W. Simon, Manual of Chemistry (Henry C. Lea’s son & Co. Philadelphia, 1884), p. 291.
(13) http://tera-3.ul.cs.cmu.edu/NASD/4a7f1db4-5792-415c-be79-266f41eef20a/upgrade-archive-06-14-2007/data/upload/disk2new/COMPLETE_158256_OU_Manual_Of_Chemistry/RTF/00000495.rtf
(14) A.M. Giovannozzi, F. Rolle, M. Sega, M.C. Abete, D. Marchis, and A.M. Rossi, Food Chem.159, 250-256 (2014).
(15) L.M. Chen and Y.N. Liu, ACS Appl. Mater. Interfaces3, 3091-3096 (2011).
(16) V. Canpean and S. Astilean, Spectrochim. Acta Mol. Biomol. Spectros.96, 862-867 (2012).
(17) X. Liu, X. Wang, L. Zha, D. Lin, J. Yang, J. Zhou, and L. Zhang, J. Mater. Chem.C2, 7326-7335 (2014).
(18) R.M. Jarvis, H.E. Johnson, E. Olembe, A. Panneerselvam, M.A. Malik, M. Afzaal, P. O’Brien, and R. Goodacre, Analyst133, 1449-1452 (2008).
(19) B. Sharma, R.R. Frontiera, A.I. Henry, E. Ringe, and R.P. Van Duyne, Mater. Today 15, 16-25 (2012).
(20) A.A. Hamamat and W.W. Nawar, J. Agric. Food Chem. 39, 1063-1069 (1991).
Magdalena Wrona, Jesús Salafranca, and Cristina Nerín are with the Department of Analytical Chemistry at the Aragon Institute of Engineering Research I3A with the University of Zaragoza in Zaragoza, Spain. Massimiliano Rocchia is with Thermo Fisher Scientific in Rodano-Milan, Italy. Direct correspondence to: fjsl@unizar.es

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.