GC×GC–MS for Forensic Analysis
Special Issues
Forensic scientists often encounter highly complex analytical problems related to crime scenes that would benefit from the capabilities of GC×GC–MS. However, this technique has not been fully explored to help benefit forensic laboratories.
Gas chromatography–mass spectrometry (GC–MS) is considered the gold standard in forensic trace evidence analysis, because of its ability to chromatographically separate and analyze components in mixtures. Although two-dimensional GC–MS (GC×GC–MS) has been used extensively in the oil and petroleum and flavor and fragrance industries, it has not been fully explored in the forensic sector. However, forensic scientists often encounter highly complex samples that would benefit from the capabilities of GC×GC–MS, such as sexual lubricants, automobile paints, and tires. GC×GC–MS analysis can allow for the deconvolution of coeluted components, while providing increased sensitivity of minor components to help benefit any forensic laboratory.
Gas chromatography–mass spectrometry (GC–MS) is a "go-to" analytical technique, primarily because of its versatility for isolating and analyzing different components in unknown mixtures, without requiring substantial method development for each new sample. This is the primary reason why GC–MS is the gold standard in the forensic analysis of trace evidence, such as ignitable liquids and drugs. However, there are limitations in using GC–MS for all unknown mixtures, because of the complexity of some of these mixtures. The primary limitation is coelution of the compounds in a mixture. This is where multidimensional gas chromatography (MDGC) can increase component separation with the potential to increase the sensitivity of compounds that may not meet the limit of detection in GC–MS. There are a few types of multidimensional gas chromatography configurations, all of which can be coupled to a mass spectrometer: comprehensive (GC×GC–MS) or heart-cut (GC–GC–MS). There are three types of commercially available MDGC–MS configurations: thermal modulation (TM), Deans switch (DS), and differential flow modulation (DFM). Discussions of TM and DS can be found in the literature (1,2). This article will discuss the use of the latter modulator for forensic trace evidence analysis to rapidly differentiate complex mixtures by observing the unique chromatographic "fingerprint" (3). These "fingerprints" are similar to a topography chart, which shows the trends of compounds that are chemically related; that is, normal alkanes and isoparaffins. As a result of increased sensitivity, this "fingerprint" shows both major components, as well as those minor components that may have been hidden as a result of coelution.
GC×GC–MS systems have been used in the edible oil industry to investigate minor compounds (3,4), as well as the petroleum and biodiesel industries for rapid determination of the chemical formulation (5). However, the technique has yet to be evaluated for complex forensic evidence. This article discusses the use of GC×GC–MS for several forensic samples.
Experimental
Both GC–MS and GC×GC–MS analysis of the trace evidence samples were performed on the same GC–MS system, using the same column configuration. The GC system was a 7890B gas chromatograph, equipped with a split–splitless injector coupled to a 5977 quadrupole mass spectrometer (Agilent) (6).
The pyrolysis analysis of automobile paint and tires used the same GC×GC–MS method from the lubricant analysis. However, to conduct pyrolysis of the sample, a Pryoprobe 4000 (CDS Analytical LLC) was used. The flash pyroprobe profile was started at 50 °C for 2 s, and then was ramped to 750 °C at 50 °C/s ,and held for 2 s. All samples were analyzed in their natural, unmodified state.
Forensic Lubricant Analysis
A recent survey conducted by the National Center for Injury Prevention and Control revealed that approximately 1 in 5 women, and 1 in 71 men, will be sexually assaulted in their lifetime (7). Despite this staggering statistic, most criminal investigators primarily rely on DNA evidence to solve these crimes-from semen, skin cells underneath fingernails, or any other biological evidence. However, the use of condoms by sexual perpetrators has increased, primarily because they think that it will mitigate the deposition of semen at the crime scene or on the victim, thus preventing their identification based on DNA. A study by Nancy Ritter demonstrated that approximately 30% of sexual assault kits do not contain any probative DNA profiles for the perpetrator (8). This is where the forensic analysis of sexual lubricants can support the current analysis of sexual assaults. In the absence of DNA, lubricant analysis can provide another link between the perpetrator and the victim or crime scene. However, many lubricants are made from natural oils, which are comprised of many compounds that may be difficult to differentiate using traditional GC–MS.
An example of a typical oil-based organic personal lubricant is one comprised of several organic butters (cocoa and shea), as well as vitamin E oil, beeswax, sweet almond oil, and even sunflower oil. Each of these oils and butters are comprised of many different oils and components themselves. Lubricant samples were prepared by hexane solvent extraction. Despite the fact that the oil-based lubricant only has six labeled ingredients, GC–MS analysis shows that there were more than the six labeled components, but there was a substantial amount of coelution between retention times (tRs) of 7 and 20 min (Figure 1a). However, using GC×GC–MS analysis, more than 25 different components were readily observed. Between the 10 and 15 min first dimension retention times (FDRTs), several components were separated in the second dimension that were coeluted during GC–MS analysis (Figure 1a).
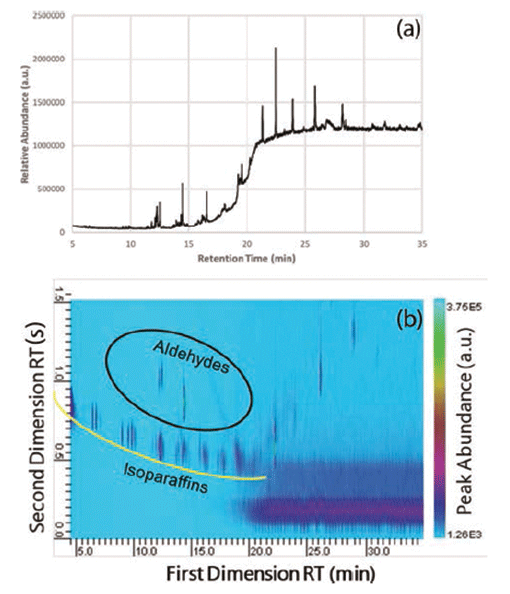
Figure 1: (a) GC–MS and (b) GC×GC–MS of an oil-based lubricant.
When compared to other natural oil-based or plant-based lubricants, the overall chromatographic profile is similar, but the differences are readily observed between the FDRTs of 7 and 17 min. Isoparaffinic compounds make up the lower arc of the early GC×GC profile (underlined in yellow) and the aldehydes are above (circled in black). Many of the heavier oils elute later on the first-dimension column, such as vitamin E oil. This oil is not readily observed in this sample, primarily because of the low concentration in the sample. Based on the analysis of other natural lubricants and lotions, vitamin E (also known as a-tocopherol) elutes off the second column adjacent to the column bleed located at the lower right-hand corner of the chromatographic plane. What is also immediately noticeable is the increased intensity.
It was not immediately clear why there was a background shadow observed between first dimension retention times 20 to 35 min (lower right hand of Figure 1b). It is possible that this "shadow" was a result of either a column bleed from either the first-dimension or second-dimension column, considering the elevated oven temperatures at the end of the chromatograph run (280 °C).
Automotive Paint Analysis
Automotive paint is a type of forensic evidence collected at car accidents, hit-and-runs, and any other crime involving a vehicle. This type of evidence is encountered frequently, and thus it is critical to improve current analytical techniques, as well as evaluate new options that could provide more information than current techniques can provide.
Automotive paint is chemically complex because it is a multilayer system and different components are present in each layer. The four main components that make up automotive paint are pigments, additives, binders, and solvent. When automotive paint coatings are applied by the original equipment manufacturer (OEM), they are added in the following order: electrocoat, primer surfacer, basecoat, and clear coat. Each of the coatings have a different purpose with regards to the car's appearance. The electrocoat is used to prevent corrosion and the primer surfacer provides the car with a smooth surface. The basecoat determines the color of the vehicle, and the glossy finish is provided by the clear coat, which contains hindered amine light stabilizers and UV absorbers to protect the underlying paint layers from weathering and environmental effects (9).
Currently, there are three techniques used to analyze automotive paint: microscopy, infrared (IR) spectroscopy, and pyrolysis (py)-GC–MS. Py-GC–MS has the most discriminating power among these three techniques, and can differentiate between samples with similar binders and pigments, not typically achievable with IR spectroscopy (10). The ability of py-GC–MS to discriminate between similar samples is significant, yet there is still room for improvement. Pyrograms of automotive clear coat samples analyzed using py-GC–MS have indicated that coelution occurs with certain compounds of interest; that is, toluene and 1,2-propandial, which can limit the ability to differentiate clear coats (10).
To overcome the obstacle of coelution, py-GC×GC–MS was used to analyze automotive clear coats. To our knowledge, there is no literature published on the analysis of paints using py-GC×GC–MS. Increased separation of paint components is demonstrated using py-GC×GC–MS, especially for peaks that typically coelute in GC–MS. The two peaks around FDRT 11.6 min (Figure 2b) illustrate the improved separation that is achieved in py-GC×GC–MS. α-methylstyrene (11.776 min FDRT) and n-butyl methacrylate (11.600 min FDRT) would normally coelute in the first column. However, the second column allows the two peaks to be distinguished from one another. With additional method development, we aim to increase the separation of clear coat peaks.
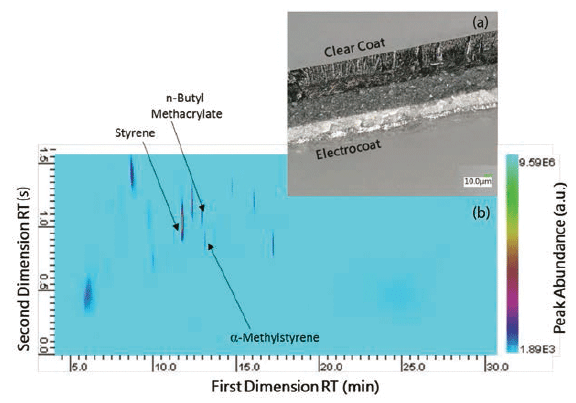
Figure 2: (a) Cross-section of automotive paint system, (b) Py-GC×GC-MS profile of the clear coat.
Tire Analysis
Much like automotive paint, traces of tire rubber are often encountered on road surfaces or on the victim of automotive-related incidents, such as hit-and-run accidents. The forensic analysis of tire evidence is useful to investigators, specifically when attempting to reconstruct vehicle trajectories, velocities, and dynamics in incidents (11). Tire impressions from a crime scene are routinely compared to the tread pattern of tires from the suspect vehicle. However, in many instances the impression may be of poor quality, which is when the chemical analysis of the rubber traces may help to provide investigative leads. The physiochemical complexity of trace tire particulates makes the characterization of this evidence challenging and time-consuming. Py-GC–MS is the technique primarily used by forensic scientists for the chemical analysis of tire evidence (12,13). The pyrograms from rubber traces obtained from the tire impressions can then be compared to the tire from a suspect vehicle. Tires are extremely chemically complex, often containing over 200 components, including natural and synthetic rubber, oils, plasticizers, antioxidants, antiozonants, accelerators, vulcanizing agents, and curing systems (14). This chemical complexity can result in coelution of components, which may prevent a correct match and lead to significant errors.
A flash pyrolysis method was used to pyrolyze a small portion (~50 µg) of the main tread of a Firestone Destination LE tire. Multidimensional separation of the pyrolysates was performed, and the resultant GC×GC plot is presented in Figure 3. The complexity of tire samples makes identification of the individual components difficult using one-dimensional py-GC–MS. Py-GC×GC–MS was able to differentiate many components in the second dimension, which is beneficial to eliminate the ambiguity in making comparisons, and improves match determinations and reduces errors, which is imperative in forensic investigations.
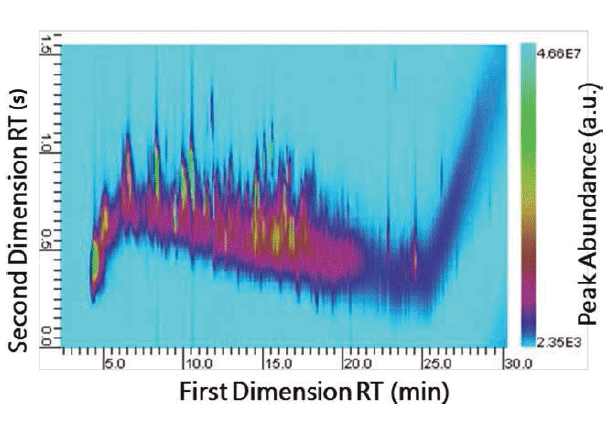
Figure 3: Py-GC×GC–MS profile of a tire sample.
Conclusions
With complex mixtures commonly encountered in forensic trace analysis, it is necessary to start evaluating techniques other than GC–MS. The use of GC×GC–MS or py-GC×GC–MS provide the forensic community with a new methodology that can achieve such separation. This could be the next frontier for increasing the actionable intelligence that forensic laboratories provide the criminal investigation system.
Acknowledgments
The authors would like to thank Drs. Matthieu Baudelet and Mauro Martinez from the National Center for Forensic Science for their assistance on the tire project. The lubricant analysis research was funded by the National Institute of Justice [Award: 2016-NE-BX-0001]. The automobile paint research was supported by the American Academy of Forensic Science's Forensic Science Foundation Lucas Grant.
References
(1) M. Adahchour, J. Beens, R.J.J. Vreuls, and U.A.T. Brinkman, Trends Analyt. Chem. 25, 540–553 (2006).
(2) K.M. Sharif, S.-T. Chin, C. Kulsing, and P.J. Marriott, Trends Analyt. Chem. 82, 35–54 (2016).
(3) G. Purcaro, L. Barp, M. Beccaria, and L.S. Conte, Food Chem. 212, 730–738 (2016).
(4) M. Biedermann, A. Bongartz, C. Mariani, and K. Grob, Eur. Food Res. Technol. 228, 65–74 (2008).
(5) S. Castillo, I. Mattila, J. Miettinen, M. Oresic, and T. Hyotylainen, Anal. Chem. 83, 3058–3067 (2011).
(6) C. Bridge and M. Giardina, J. Chromatogr. A Submitted (2018).
(7) M.C. Black, K.C. Basile, M.J. Breiding, S.G. Smith, M.L. Walters, M.T. Merrick, J. Chen, and M.R. Stevens, The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. N.C.f.I.P.a. Control (Centers for Disease Control and Prevention, Atlanta, Georgia, USA, 2010), pp. 17–26.
(8) N. Ritter, NIJ Journal 270, 4–17 (2012).
(9) E.A. Liszewski, S.W. Lewis, J.A. Siegel, and J.V. Goodpaster, Appl. Spectrosc. 64, 1122–1125 (2010).
(10) J. Zieba-Palus, G. Zadora, J.M. Milczarek, and P. Koscielniak, J. Chromatogr. A 1179, 41–46 (2008).
(11) M.B., H.-g. Xu, Y. Chen, and M.-y. Lin, Adv. Mech. Eng. 9, 1–13 (2017).
(12) J. Ding and H. Liu, Forensic Sci. Int. 43, 45–50 (1989).
(13) G. Sarkissian, J. Forensic Sci. 52, 1050–1056 (2007).
(14) A. Evans and R. Evans, The Composition of a Tyre: Typical Components (The Waste & Resources Action Programme, Banbury, United Kingdom, 2006).
Candice Bridge, PhD is an assistant professor in the department of chemistry and a research professor at the National Center for Forensic Science at the University of Central Florida. Mark Maric, PhD is a postdoctoral associate at the National Center for Forensic Science. Kaitlin Jones is a graduate student at the University of Central Florida, and conducts automobile paint analysis at the National Center for Forensic Science. Direct correspondence to: candice.bridge@ucf.edu

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.