An Interlaboratory Comparison Study for the Determination of Arsenic and Arsenic Species in Rice, Kelp, and Apple Juice
Spectroscopy
An interlaboratory comparison study for the measurement of arsenic species in rice, kelp, and apple juice was carried out. The purpose of the study was to enable participating laboratories to evaluate their analytical capability to determine inorganic arsenic, arsenite, arsenate, monomethylarsonic acid, and dimethylarsinic acid, assess the intercomparability of the data generated, and look for any correlation trends between the results and the analytical procedures used.
An interlaboratory comparison study for the measurement of arsenic species in rice, kelp, and apple juice was carried out. The purpose of the study was to enable participating laboratories to evaluate their analytical capability to determine inorganic arsenic, arsenite, arsenate, monomethylarsonic acid, and dimethylarsinic acid, assess the intercomparability of the data generated, and look for any correlation trends between the results and the analytical procedures used. Approximately, 66% of laboratories achieved an overall score of 3 (good) or 4 (outstanding), based on the absolute Z-value statistical analysis procedure, indicating that there was generally good agreement between the labs reporting arsenic speciation data for food matrices. However, only 15% of laboratories achieved an overall score of 4, which could be indicative that further method development needs to be carried out to achieve a higher level of consistency across multiple laboratories worldwide using different analytical procedures.
This interlaboratory comparison study for the determination of arsenic and arsenic species in rice, kelp, and apple juice was initiated to provide a reliable means for laboratories to evaluate their competency in the analysis of arsenic species in these matrices, as well as a metric for assessing the intercomparability and validity of the data generated by different laboratories. An additional objective of the study was to look for any correlation trends between the matrices analyzed, species determined, and the analytical procedures used by the different participating laboratories.
The study materials included white rice flour, brown rice flour, kelp powder, and apple juice. Participants were asked to analyze the samples for arsenic (As) species using the methods that are commonly used in their laboratory. Participants were asked to report results for as many of the following analytes as possible, based on their analytical methodology: total As in the sample, total arsenic in the speciation extract (if applicable), inorganic As, As(III), As(V), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA). Participants were asked to measure and report the total arsenic concentration in the sample and in their speciation extract for the purpose of determining extraction efficiency.
Some of the key features of the study were a broad invitation to participate; no participation fee; a large group of participating laboratories from around the world; anonymous data submission, analysis, and reporting; and the inclusion of analytical method reporting. In total, 46 laboratories from 15 countries registered to participate and were sent the study materials, and 41 datasets were received from 39 laboratories (two of the laboratories submitted datasets by two different methods).
Materials and Methods
Samples Studied
Four materials were studied, as described here:
White and Brown Rice Flour
Milled white rice flour and brown rice flour were provided by the Dale Bumpers National Rice Research Center (part of the U.S. Department of Agriculture [USDA] Agricultural Research Service) in Stuttgart, Arkansas. The rice flours were grown in the United States and were identified as "2012 Stuttgart, AR/USDA ARS CLXL745 Row Irrigation 40% FC." The rice flours were analyzed before use in this study, and the total arsenic concentrations met our criteria for use of greater than 5 ppb.
Kelp Powder
The kelp powder (Laminaria digitata) was purchased commercially from the bulk foods section of a natural food co-op in Seattle, Washington. This organic product was harvested from a remote bay off the northwest coast of Iceland. According to the manufacturer, the kelp was grown deep in the tidal zone, away from the shipping lanes, where it would hypothetically not be exposed to the pollutants present in more industrialized areas. The kelp powder was analyzed before use in the study, and was found to be high in total arsenic (approximately 50–100 ppm).
Apple Juice
The apple juice was purchased commercially from a grocery store in Seattle, Washington, and was 100% pure juice from apples grown in the United States. The juice was analyzed before use in this study, and the total arsenic concentration met our criteria for use of greater than 5 ppb.
Methodology
The rice flour and kelp powder samples were thoroughly homogenized, and aliquots were placed in 50-mL homopolymer polypropylene vials. Juice samples were distributed into 125-mL high-density polyethylene (HDPE) bottles. Both the vials and the bottles were pretested and found to be from a lot that was low in total arsenic. Vials and bottles were individually double-bagged in zip-type bags and stored in cardboard shipping boxes.
All samples were shipped to the participating laboratories during the first two weeks in April 2013. Participating laboratories were asked to analyze samples for total arsenic and arsenic species in accordance with their standard operating procedures and were given no further guidance on analytical methodology, although they were also asked to supply details of their standard methodology. However, many of the laboratories that used inductively coupled plasma–mass spectrometry (ICP-MS) for carrying out total As determinations also cited two well-established publications that coupled ICP-MS to high performance liquid chromatography (HPLC) for the determination of As species, as the basis for their speciation analytical methodology (1,2).
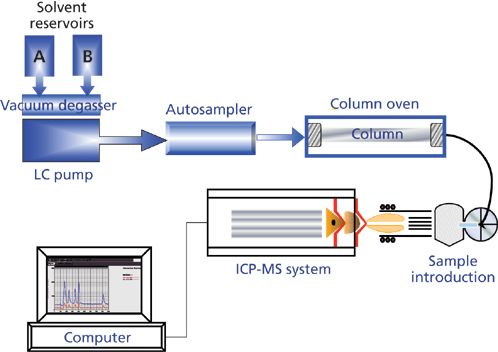
Figure 1: A typical configuration for HPLC coupled with an ICP-MS system.
The vast majority of participating laboratories in this study used the HPLC–ICP-MS approach for carrying out the analyses. This trace elemental determination of arsenic species in biological samples has been used with great success for more than 20 years (3,4). Some of the early research showed that it was possible to separate and detect extremely low levels of both inorganic and organic arsenic compounds by coupling an HPLC system to an ICP-MS. In this technique, the species of interest are separated from one another by injecting the sample matrix into an HPLC system and then passing the separated eluent into the ICP-MS system for detection. Through optimization of the chromatographic separation procedure, column material, elution parameters, and ICP-MS operating conditions, the different species can be separated to produce a time-resolved elemental species chromatograph, where the separated peaks can be detected, measured, and quantified. A typical configuration for an HPLC–ICP-MS system is shown in Figure 1. A time-resolved chromatogram of five As species - arsenobetaine (AsB), dimethylarsinic acid (DMA), As(III), monomethylarsonic acid (MMA), and As(V) - is shown in Figure 2.
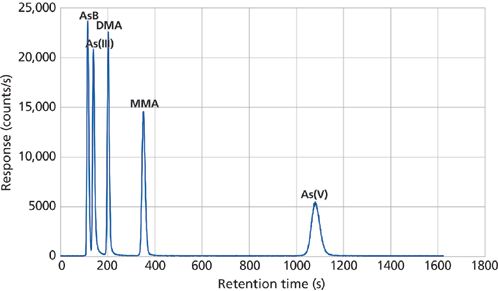
Figure 2: An example of a time-resolved HPLC–ICP-MS chromatogram of five 10 ppb arsenic species standards using a Hamilton PRP X100 column.
Participants were asked to report the following results, or subset of results based on their methodology:
- Total arsenic (As) in the sample
- Total As in the extract (if applicable)
- Inorganic As
- As(III) – arsenite
- As(V) – arsenate
- MMA – monomethylarsonic acid
- DMA – dimethylarsinic acid
Other arsenic species, such as AsB, or unknown arsenic species were also reported by some laboratories.
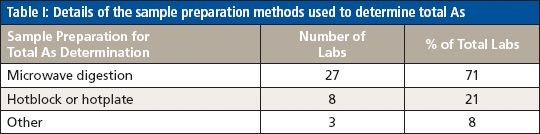
Table I: Details of the sample preparation methods used to determine total As
All results were reported to an independent third party, EcoChem, Inc., which is a data validation company that had no role in the study other than data management. At EcoChem, the dataset was compiled, and a unique identifier was assigned to each laboratory before it was transmitted to Brooks Rand Labs. Following the release of the study results (6), each participating laboratory received its own unique identifier, but identifiers were not disclosed to any other parties, including Brooks Rand Labs. This research design ensured there would be no bias by the preparers of this report against any participating laboratory and that participants could submit data with the comfort of anonymity.
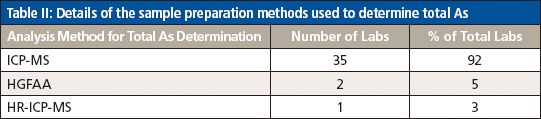
Table II: Details of the sample preparation methods used to determine total As
Equipment Used for the Study
For the analysis of total As, the majority of participating laboratories used microwave digestion and ICP-MS, while two of the laboratories used heated graphite furnace atomic absorption (HGFAA) and one laboratory used high-resolution (HR) ICP-MS. Tables I and II give details of the sample preparation and analysis methods used to determine total As. Tables III, IV, and V give a breakdown of the As speciation extraction, separation, and detection methods, respectively.
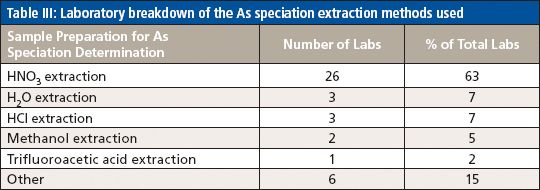
Table III: Laboratory breakdown of the As speciation extraction methods used
Data Analysis and Calculations
Each laboratory was asked to report an analytical result, detection limit, and date of analysis for each sample and analyte. These data were the basis of the scoring used to evaluate the intercomparison study. In addition, each laboratory reported information on the sample preparation, extraction method, separation technology, analytical methodology, and equipment used. These data were used to compile assessments of the performance of various analytical methods, but were not used in laboratory scoring.
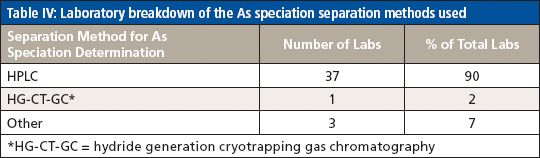
Table IV: Laboratory breakdown of the As speciation separation methods used
Statistical data analysis was performed following the method favored by the United States Geological Survey's (USGS) Standard Reference Sample Project, with data evaluation using nonparametric statistics (5). This statistical approach was chosen because it is median-based and therefore unaffected by the influence of outliers.
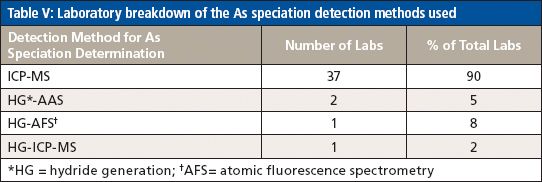
Table V: Laboratory breakdown of the As speciation detection methods used
The absolute Z-value for each result is calculated using the following equations:

where Z = the absolute Z-value assigned to each result for the purpose of assigning a rating; X = the reported value; M = the median value reported by all laboratories (excluding values below the reported detection limit); F = the F-pseudo-sigma (approximates the standard deviation of traditional statistics when the data has a Gaussian distribution); calculated by dividing the interquartile range (or fourth-spread) by 1.349. The 1.349 value is derived from the number of standard deviations that encompasses 50% of the data; and Q = the interquartile range (the difference between the first quartile and third quartile of a set of data).
Participating laboratories were requested to report undetected (u) values as being less than their detection limit. If a value was less than that laboratory's reported detection limit, then that value was omitted and the "u" qualifier was added. For these samples, performance was not rated, with the following exception. If the laboratory's detection limit was less than the median value reported by all laboratories and had a Z-value greater than 2, then the laboratory would receive a rating of 0 for that analyte (potential false negative).
To assign a score to each laboratory's performance, ratings were assigned based on each laboratory's absolute Z-value for each analyte, as listed in Table VI.
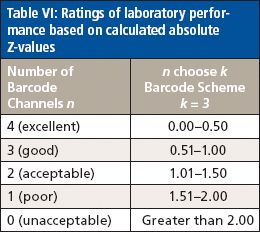
Table VI: Ratings of laboratory performance based on calculated absolute Z-values
Scores were not assigned if the overall number of data points for the analyte and sample in question (omitting values that were less than the reported detection limit) was less than seven or when the calculated F-pseudo-sigma value (F) was greater than the median value (M).
Analytical Results
The results reported by each laboratory for each of the material types can be found in the final Brooks Rand Labs report (6), along with the median (M) value, the mean value, the F-pseudo-sigma (F) value, and number of laboratories reporting results above their detection limit (n) for each parameter.
The complete data set will not be reported in this article, but a summary of the M values for each analyte are listed in Table VII. Values in parentheses were associated with F values that were greater than the M values, or with datasets with less than seven results; therefore, the variability in the data was too high or the number of results was too small to use the M value as a consensus value for the purpose of scoring the individual laboratories' results. A reported result of "ND" means that all reported values were less than their associated detection limits. It should also be noted that two laboratories reported measuring unidentified arsenic species at levels above their detection limit in the kelp powder sample.
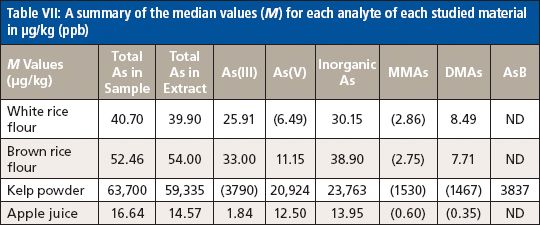
Table VII: A summary of the median values (M) for each analyte of each studied material in μg/kg (ppb)
The arsenic speciation results as a fraction of the total arsenic in the extract are shown in Figure 3. For the rice samples, the inorganic arsenic made up about 80% of the total arsenic in the extract, with the sum of the species totaling just over the result for total arsenic in the extract (shown as >100%). This is consistent with inorganic arsenic results reported in the literature (7,8). For the kelp powder sample, the sum of the species totaled approximately 50% of the total arsenic found in the extract, indicating that there were forms of arsenic present that were extracted but not determined by the analytical techniques used. With the apple juice extract, nearly 95% of the arsenic found was in the inorganic form.
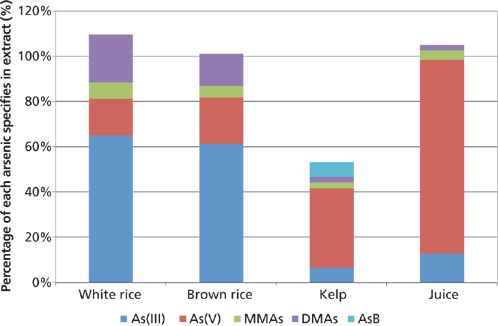
Figure 3: The mean arsenic speciation results for each matrix as a fraction of the total arsenic in the extract.
Participating Laboratories
Of the 47 laboratories that agreed to participate in this study, 46 of them received the study materials; one laboratory in Mexico was not able to import the materials. Of the 46 laboratories that received the study materials, 41 datasets were received from 39 laboratories (two laboratories reported data by two methods). The list of the participating laboratories can be found in the final report. Of the participating laboratories, only 11 laboratories reported all of the requested parameters in every material type. Nearly half of the laboratories (20 out of 41) did not report results for both total arsenic in the sample and total arsenic in the extract; therefore, extraction efficiencies for those laboratories could not be calculated. In fact, four of the laboratories (numbers 6, 19, 26, and 27) did not report any total arsenic data.
Of the 39 participating laboratories, approximately one-third of them were from outside of North America. Of the North American participants, all were from the United states, with the exception of one laboratory from Canada. Of the laboratories from the United States, nine (38%) were private commercial or industry testing laboratories, eight (33%) were state laboratories representing seven states, five (21%) were laboratories associated with the U.S. Food and Drug Administration (FDA), and two (8%) were university laboratories. There were eight participating laboratories from Europe (21%) representing five countries. In addition, there were four participants from Asia and one each from Australia and New Zealand. Table VIII summarizes the regional participation in this study.
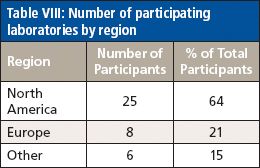
Table VIII: Number of participating laboratories by region
Laboratory Performance Ratings
In total, the participating laboratories generated results comprising 546 data points, which were used for scoring. The mean scores for the various analytes were relatively consistent, with an overall score for the study of 2.6, and with mean scores ranging from 2.6 for the total As in the sample to 2.9 for the DMA. This can be seen more clearly in Table IX.
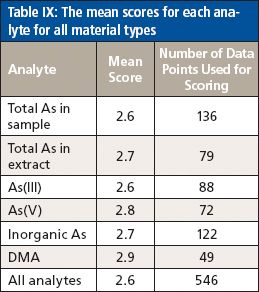
Table IX: The mean scores for each analyte for all material types
Similarly, the mean scores for the sample matrices were also relatively consistent, ranging from 2.5 for the brown rice flour to 2.9 for the kelp powder, as shown in Table X.
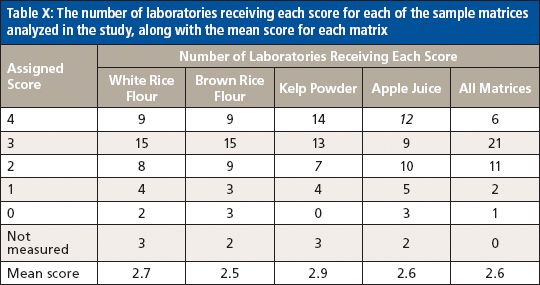
Table X: The number of laboratories receiving each score for each of the sample matrices analyzed in the study, along with the mean score for each matrix
Figure 4 groups the participating laboratories based on the performance scores for each of the studied materials. The distribution of average scores for these four matrices were quite similar, with an average score of 2.7 for the white rice flour, 2.5 for the brown rice flour, 2.9 for the kelp, and 2.6 for the apple juice. The scores for all matrices combined are summarized in Figure 5. These data generated an overall mean score for the study of 2.6.
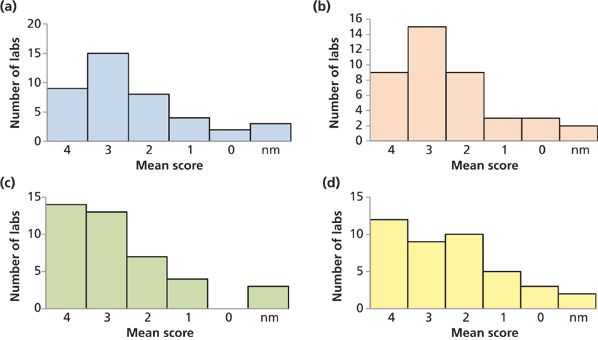
Figure 4: Numbers of laboratories per mean score for the four samples studied. Note: nm = not measured.
It should be emphasized that the majority of laboratories participating in this study (66%) achieved an overall score of 3 or 4 (good or excellent) indicating that there is generally good intercomparability amongst most laboratories that are reporting total arsenic and arsenic speciation data for these matrices. In total, 93% of the laboratories received an overall score of 2 (acceptable) or better, and only two laboratories (7%) received scores of 1 or 0 (poor or unacceptable). However, with only six laboratories (15%) receiving an overall score of 4, it is clear that further analytical method development is required to achieve a high level of consistency across multiple laboratories using various methods worldwide.
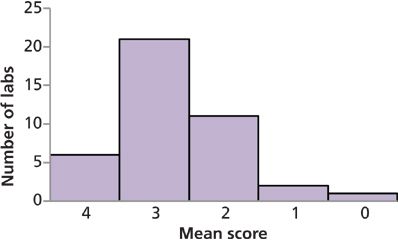
Figure 5: Numbers of laboratories per mean score for all test matrices combined.
It's also important to keep in mind that the studied matrices do not have "true" or "assigned" values because they are not certified reference materials. The median of all results was used as the most probable value (M-value) for each analyte. Therefore, if many laboratories used the same method, and that method is prone to errors from species conversion or species coelution, then the resulting M-values have the risk of being biased. This is exemplified by the fact that many laboratories used a nitric acid sample extraction for the speciation analyses, which has the potential to cause oxidation of some or all of the As(III) species to As(V) (2). In addition, when extracts of samples containing significant levels of arseno-sugars or AsB (for example, sea plants) are analyzed on some LC columns, there is a strong possibility of coeluted As(III), AsB, and other large organic arsenic molecules such as dimethylarsinic acid (DMA) (2). For that reason, if the appropriate steps are not taken, these types of interferences will most likely produce erroneous results. This is exemplified in the chromatogram in Figure 6, which shows that the first eluted peak is probably an overlap of AsB, DMA, and As(III). The second small peak is monomethylarsonic acid (MMA), while the final peak eluted peak is As(V).
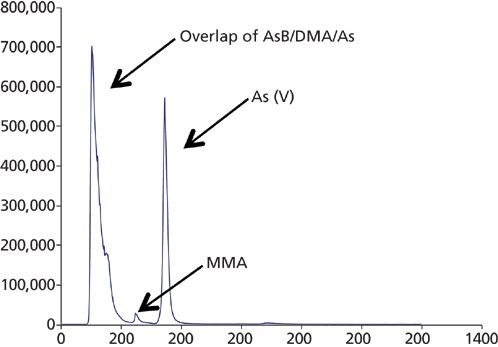
Figure 6: Chromatogram of the separation of different arsenic species on an ESI CF-Cr-01 column, showing the AsB, DMAs, and As(III) being coeluted (chromatogram provided by unidentified participating laboratory).
Results and Discussion
Extraction Efficiency
For the determination of arsenic species in any solid food matrix, a successful extraction method must meet three important criteria:
- It must be capable of extracting all the arsenic species from the matrix.
- It must preserve both oxidation and complexation states, unless only the inorganic species are of interest.
- It must be able to produce a solution that is compatible with the separation and analytical methodologies.
Typically, samples are prepared for total arsenic determination using aggressive dissolution reagents that completely liberate all arsenic from the matrix. However, for speciation analysis, less aggressive reagents have to be used to preserve oxidation and complexation states. Different extractant or matrix combinations can have very different extraction efficiencies. Therefore, it is important to compare the total amount of arsenic found in the extract for speciation analysis to the total amount of arsenic in the sample. As previously stated in this study, the majority of the laboratories used a dilute nitric acid extraction method for the preparation of the speciation samples. To assess the extraction efficiency of the sample preparation method used in this study, laboratories were asked to measure the total arsenic concentration in both the sample and the speciation extract. The total arsenic extraction efficiency was then calculated by dividing the result for total arsenic in the extract by the result for total arsenic in the sample. The extraction efficiency for the two rice samples and one kelp sample for each laboratory were then averaged, and the results are shown in Figure 7.
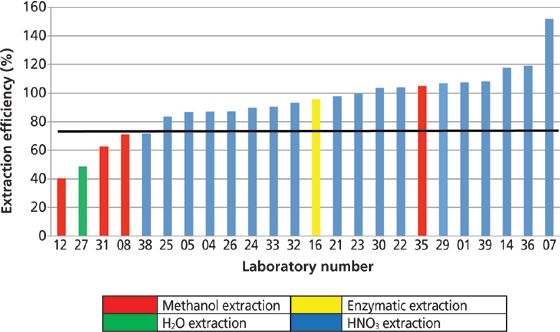
Figure 7: The mean extraction efficiency for total arsenic in the solid samples (rice and kelp samples), with extraction methods indicated by pattern and the mean extraction efficiency indicated by the solid black line.
It should be noted that only 59% of the laboratories reported extraction efficiency data. The mean extraction efficiency for all results reported for the solid samples was 93 ± 23%. An indication of the extraction method used by each laboratory is provided by the pattern of the bar in Figure 7. The majority of the laboratories that reported results for both total arsenic in the sample and total arsenic in the extract used some form of nitric acid extraction method (blue). Many of these laboratories followed the procedure described by the FDA (2). With the exception of the highest and the lowest value, all extraction efficiencies for samples prepared with a nitric acid extraction method were between 80% and 120%. In contrast, with the exception of one laboratory, extraction efficiencies for samples prepared with methanol or water extraction were typically much lower (40–70%). In reviewing the detailed descriptions of the sample preparation methods provided by the laboratories, only one laboratory used microwave heating in conjunction with the methanol extraction, which may account for the improved extraction efficiency.
The impact of the extraction procedure can be exemplified by taking a closer look at the brown rice and kelp sample matrices.
Brown Rice
The results and characteristics for white rice flour and brown rice flour were found to be very similar; therefore, the white rice data are not shown separately in this section.
The median result for As(III) in brown rice was found to be 33 μg/kg. This is exemplified in Figure 8, which shows inorganic arsenic values displayed by extraction method. The laboratories that reported As(III) results within 20% of the median value were well distributed between those that used methanol, water, enzymatic, and nitric acid extraction methods. However, only 12 laboratories reported As(V) results at quantifiable levels, while seven did not report As(V) values at all. Also, 22 of the laboratories actually reported results that were below their detection limits. Most of the laboratories that reported As(V) used a nitric acid digestion technique. The median value of the As(V) results that were quantifiable was 11 μg/kg. However, the relative standard deviation (RSD) was 70% from those reporting laboratories. For this reason, it is very difficult to assess the true value of As(V) in the brown rice sample, with such a large number of nondetected results.
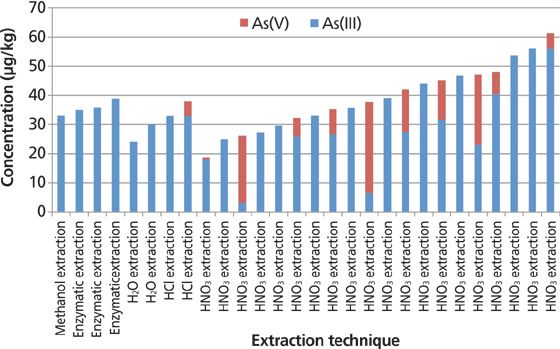
Figure 8: Inorganic arsenic values in brown rice displayed by extraction method.
The MMA results in rice were negligible, with very few laboratories reporting results, and because all the reported results were at or near their detection limits, these data are not shown. It is also important to emphasize that only half of the laboratories participating in the study were able to quantify DMA in rice with a median concentration of 7.7 μg/kg. Most laboratories that reported DMA in the rice flour used a nitric acid digestion. The DMA data in brown rice are shown in Figure 9.
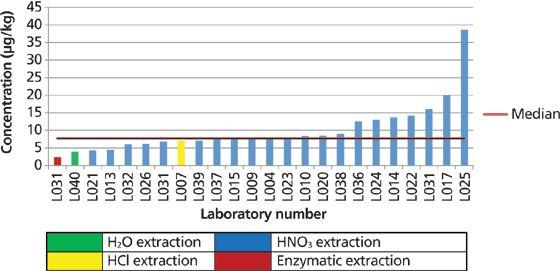
Figure 9: DMA in brown rice displayed by laboratory number and extraction procedure.
Kelp
The kelp was included in the study because of the recognized high level of arsenic typically associated with this matrix. In addition, there are many less common organic arsenic species found in kelp. The DMA results in particular varied widely with a range of 331–37,068 μg/kg. Of the 23 laboratories that reported DMA, nine laboratories reported results of less than 1000 μg/kg, while 10 laboratories reported results in the 1000–10,000 μg/kg range, and four reported results over 10,000 μg/kg. The MMA results also varied widely with a range of 21–48,186 μg/kg. Of the 18 laboratories that reported MMA, eight reported results of less than 1000 μg/kg, five reported results in the 1000–10,000 μg/kg range, and five laboratories reported results over 10,000 μg/kg. The DMA and MMA results for the individual laboratories displayed by extraction technique are shown in Figures 10a and 10b (note: graphs use a logarithmic scale).
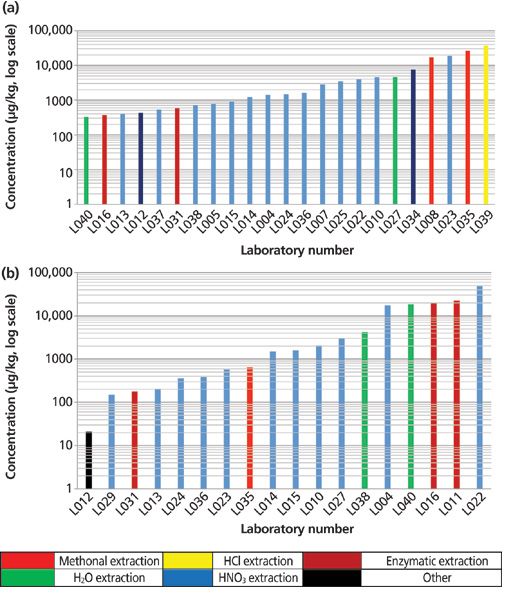
Figure 10: (a) DMA and (b) MMA results for the kelp powder sample for the individual laboratories, displayed by extraction technique (note: graphs use a logarithmic scale).
Chromatographic Separation Procedures
As mentioned earlier, after arsenic is extracted from the solid matrices, most participating laboratories used a method in which the oxidation states are separated by liquid chromatography and then quantified using ICP-MS. The challenges of this technique are achieving good chromatographic separation of the individual species, preventing coelution, and preventing shifting retention times because of matrix effects. To better understand the impact of the different separation technologies used, the kelp and apple juice sample matrices are further elucidated upon.
Quantification of Kelp
In this study, 24 of the participating laboratories used a Hamilton PRP-X100 column (Hamilton Company), and of those, most laboratories followed the FDA 4.11 method. According to the FDA method, the Hamilton PRP-X100 series column is prone to coelution and interferences at or near the retention time of the As(III) peak for species such as AsB, as described earlier. The method uses an oxidizing check to convert As(III) to As(V), which is eluted at a much later, interference-free retention time. The retention time for As(III) is then evaluated to identify any residual interfering peaks. Furthermore, the method deliberately uses an AsB check standard as a resolution check to see if the chromatography can differentiate between AsB and As(III). However, if the sample matrix causes retention time shifts, there is a possibility of coelution and peak misidentification (a risk with many column types).
Figure 11 shows the wide range of results for inorganic As in kelp, displayed by separation and column technology. The As(V) values are more consistent, with an RSD of 40% covering a range of values of 750–20,000 μg/kg, whereas the As(III) values gave an RSD of 122% with a much broader range of nondetectable to 40,000 μg/kg. The As(III) results were disproportionately related to the separation procedure that gave high total inorganic As results. The high As(III) results could potentially have been because of peak misidentification or coelution because of the presence of AsB in the kelp.
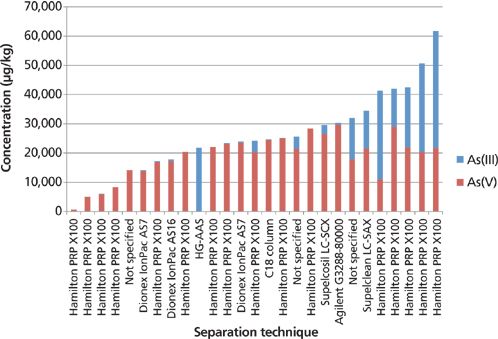
Figure 11: Wide range of results for inorganic As in kelp, displayed by separation and column technology.
Quantification of Apple Juice
In this study, 70% of the laboratories reported that they injected the apple juice directly into the HPLC column, without using any sample preparation procedure. This gives insight into the low reported method detection limits (compared to the solid samples), because of the 26 laboratories that reported As(III) in the undigested juice samples, six reported results below their detection limits, 13 reported results of 1–2 μg/kg, while seven laboratories reported results of 2–6 μg/kg. The detection limits varied, but 77% of the laboratories reported detection limits that were 1 μg/kg or lower. As(V) showed consistent results with almost 80% of the laboratories reporting results within ±1 standard deviation of the median and achieved detection limits of well below the median value. By direct injection into the HPLC system, it is shown in Figure 12 that most separation techniques compare well for inorganic As. It's also worth noting that the laboratory that used the C18 column has inverse As(III) and As(V) results compared to the median values in the study.
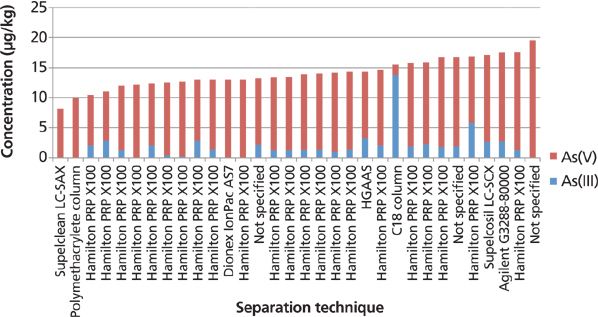
Figure 12: Inorganic arsenic in apple juice (direct injection into the HPLC column) by separation technique.
The MMA results in juice were negligible, with very few laboratories reporting results and all results near their detection limits. Only half of the laboratories reported DMA results that were above their detection limits and only a quarter were more than twice their detection limits. For this reason, these data are not shown.
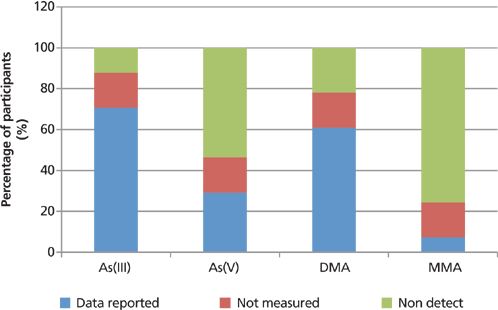
Figure 13: The variability of the detectability of the submitted speciation results for brown rice, as a percentage of the participating laboratories (note: white rice gave very similar results).
The overall variability of the detectability of the submitted results, as a percentage of the participating laboratories, is exemplified in Figures 13–15, which compare the number of participants reporting actual data with those reporting that data were below the detection capability for the studied matrices or not measured. A "nondetectable" result may be indicative of arsenic levels in the samples that were too low for the technique used for the quantification, whereas "not measured" either indicates limitations of the specific speciation technique used or the preference of the participating laboratory. It should also be noted that the white rice gave almost identical results to brown rice, with regard to the percentage breakdown of participating laboratories.
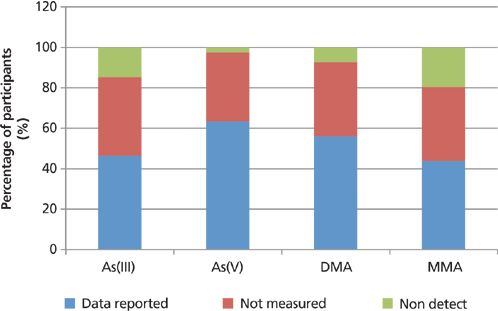
Figure 14: The variability of the detectability of the submitted speciation results for kelp powder, as a percentage of the participating laboratories.
As mentioned in the introduction, the main objective of this arsenic speciation interlaboratory comparison study was to provide a reliable means for laboratories to test their analytical procedures to evaluate their competency in the analysis of arsenic species in these food matrices. In addition, it provided an independent assessment of interlaboratory variations, which are an important metric for assessing the intercomparability and validity of the data generated by the different participating laboratories in a round-robin study. In total, 27 (66%) of laboratories participating in this study achieved an overall score of 3 (good) or better, indicating that there was generally good agreement between the laboratories reporting arsenic speciation data for these matrices. Of those 27, only six laboratories (15%) achieved an overall score of 4 (outstanding). This is probably indicative that further method development needs to be carried out to achieve a higher level of consistency across multiple laboratories worldwide using different analytical procedures.
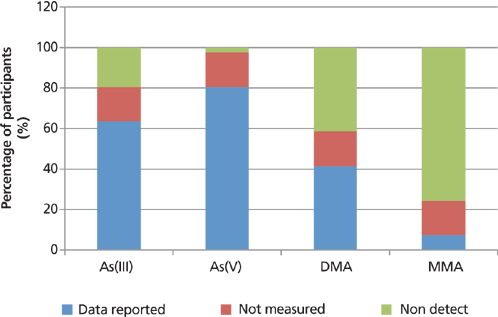
Figure 15: The variability of the detectability of the submitted speciation results for apple juice, as a percentage of the participating laboratories.
Overall Conclusions
With such a large volume of data generated, it was possible to draw some overall conclusions, based on correlation patterns between different matrices, species of interest, extraction procedures, and chromatographic separation and detection techniques used. For example, methods including a nitric acid hot block assisted extraction and a Hamilton PRP-X100 column were by far the most dominant speciation methods, used by 24 out of the 39 participating laboratories. There was no other method that was followed by more than 10% of the laboratories. The dominance of this analytical scheme, comprising over half the data reported, could possibly bias the mean values of the study to that method.
For inorganic As, which is the most important form to be measuring from a human health risk perspective, statistically valid results were achievable for all four matrices, with almost 80% of all laboratories reporting values that were within one standard deviation of the most probable value. On the other hand, most likely because of the coelution of some of the organo-As species with As(III), the data generated for kelp was extremely variable with RSD values of 40–120% for some species. This could also be related to the fact that the predominant method used is optimized for the analysis of rice products, which typically have much lower arsenic levels than kelp, which is known to contain up to 100 mg/kg (ppm).
If we take a look at individual sample matrices, the nitric acid hot block extraction of the rice samples gives a greater spread in inorganic arsenic than the combination of other sample preparation techniques. The sum of inorganic arsenic shows a smaller standard deviation than of both As(III) and As(V), possibly indicating some degree of species conversion.
The median of each species of arsenic in the kelp sample was very high, which showed a greater variability both by column and separation technique used. As(III), DMA, MMA, and AsB all had variable results potentially because of peak coelution or species conversion resulting in possible peak misidentification.
With regard to the apple juice, most laboratories did not carry out any preparation of the sample, which resulted in lower method detection limits. As(III), MMA, and DMA levels were very low in the sample, resulting in many being reported as nondetectable. And because As(V) was the major arsenic species in the juice samples and was eluted off the chromatographic column with good resolution, most separation techniques were able to quantify the arsenic species well.
This study is believed to be the largest interlaboratory comparison study for full arsenic speciation (not just inorganic arsenic) in food and juice ever conducted. As a result, individual participating laboratories should learn from it and use it to evaluate their capability compared to other laboratories in a similar field of analytical expertise. However, perhaps the overriding message from this study is that this kind of intercomparison study is an important step to identifying the current state of capabilities and the strengths and weaknesses in the methods. A similar intercomparison study will be conducted in 2014 and the results will be compared year over year. This intercomparison study clearly demonstrated that more complex matrices, such as kelp, present a challenge for the arsenic speciation methods used and steps need to be taken to enhance their robustness.
Acknowledgments
This original study and subsequent paper was made possible by the dedicated effort and input of many people. Michela Hernandez at EcoChem, Inc., received, organized, anonymized, and archived all of the data. The format of the study was based on the report for the "2012 Brooks Rand Instruments Interlaboratory Comparison Study for Total Mercury and Methylmercury" (9), authored by Joel Creswell, Virginia Engel, Annie Carter, and Colin Davies.
Many members of Brooks Rand Labs contributed to the success of this project. Annie Carter audited the accuracy of the calculations and statistical analysis that appear in this report. Samantha Dillon and Cory Wright assisted with assembling and shipping of the kits of study materials. Margaret Shultz coordinated the distribution of information and corresponded with participants as shipping issues arose. Tamas Ugrai and Christabel Escarez performed the screening analyses of more than 20 products to ensure total arsenic concentrations were suitable for this study. All funding associated with producing this intercomparison study and the associated report was provided by Brooks Rand Labs. The authors would also like to acknowledge the help of Robert Thomas of Scientific Solutions for the writing and preparation of this manuscript for publication.
Milled white rice and brown rice flour were provided by the Dale Bumpers National Rice Research Center (part of the USDA Agricultural Research Service) in Stuttgart, Arkansas. Additional logistical assistance in obtaining and shipping the rice flours to Brooks Rand Labs was provided by Agilent Technologies and the University of California, Davis.
References
(1) J. Kirby, W. Maher, M. Ellwood, and F. Krikowa, Aust. J. Chem. 57, 957–966 (2004).
(2) US Food and Drug Administration, US FDA Laboratory Methods, Elemental Analysis Manual, "Arsenic Speciation in Rice and Rice Products Using High Performance Liquid Chromatography-Inductively Coupled Plasma-Mass Spectrometric Determination," Section 4.11 (Online, FDA, Rockville, Maryland, 2012) http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm328363.htm (accessed June 11, 2014).
(3) S. Caroli, F. La Torre, F. Petrucci, and N. Violante, Environ. Sci. Pollut. Res.1, 4 (1994).
(4) J.R. Dean, L. Ebdon, M.E. Foulkes, H.M. Crews, and R.C. Massey, J. Anal. At. Spectrom. 9, 615–618 (1994).
(5) United States Geological Survey (USGS) Standard Reference Sample Project, Office of Water Quality, Branch of Quality Systems. http://bqs.usgs.gov/srs/SRS_Spr04/statrate.htm (accessed June 11, 2014).
(6) M. Briscoe, J. Creswell, and A. Carter, "Interlaboratory Comparison Study for Arsenic Speciation in Food and Juice," Brooks Rand Labs Report. (Online, 2013) http://www.brooksrand.com/wp-content/uploads/2014/02/Report+for+BRL+As+Spec+in+Food+Intercomp+2013.pdf (accessed June 11, 2014).
(7) "Arsenic in Rice: Full Analytical Results from Rice/Rice Product Sampling," US FDA Final report. www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/Metals/ucm319916.htm (accessed June 11, 2014).
(8) Consumer Reports Magazine, "Arsenic in your food: Our findings show a real need for federal standards for this toxin," http://www.consumerreports.org/cro/arsenic1112.htm (accessed June 11, 2014).
(9) J. Creswell, A. Carter, V.L. Engel, J.A. Metz, and C.A. Davies, Water Air Soil Pollut.226, 128 (2015) DOI 10.1007/s11270-015-2313-x.
Michelle L. Briscoe is the President/CEO of Brooks Rand Labs in Seattle, Washington. Tamas M. Ugrai is a metals research chemist at Brooks Rand Labs. Annie T. Carter is the VP of Operations at Brooks Rand Labs. Joel Creswell is the Director of Research and Development at Brooks Rand Instruments. Direct correspondence to: michelle@brooksrand.com

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.