Multi-Wavelength Confocal Raman Microscope for Nondestructive Pharmaceutical Ingredient Analysis
The characterization of the active pharmaceutical ingredient (API) and its distribution and physical properties in commercial medicine is necessary in drug research and development process in the pharmaceutical industry.
The characterization of the active pharmaceutical ingredient (API) and its distribution and physical properties in commercial medicine is necessary in drug research and development process in the pharmaceutical industry. Among various analytical techniques employed for this purpose, Raman spectroscopy is gaining more popularity due to its advantages as nondestructive, noninvasive, fast spectrum acquisition in seconds, high reproducibility and so on.
Fluorescence, a type of broad emission thousands times stronger than Raman effects, is very common in pharmaceutical samples such as API and final drug. It is much more likely and intense when illuminated by short wavelengths. 1064 nm excitation, as a fundamental way to avoid the fluorescence, used to be only available in FT-Raman, which is typically more complicated and slower than dispersive Raman systems. But now, BaySpec's new dispersive 1064 nm Raman spectrometer family of instruments offers users a turn-key solution that combines the speed, sensitivity, and rugged design of dispersive Raman instruments with same fluorescence avoidance of traditional FT-Raman.
The Raman spectra of the Zantac® tablet collected through the coating by Raman Microscope with 532nm, 785 nm and 1064 nm excitation laser as in Figure 1. Even for 785 nm, the fluorescent from the pigments in the coating dominated the whole spectrum and no distinguishable Raman features can be found. But with the advantages of superior fluorescence avoidance and minimal signal loss through the coating for 1064 nm dispersive Raman, a clear Raman spectrum is generated.
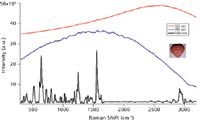
Figure 1: Raman spectra of the Zantac® tablet collected through the coating with Nomadic⢠Raman microscope excited by 532 nm, 785 nm, and 1064 nm laser.
Equipped with highly efficient VPG® gratings and innovative, deep-cooled CCD or InGaAs detector, the BaySpec's Nomadic™ Raman microscope can provide Raman spectra of all three wavelengths with high optical efficiency, high signal-to-noise ratio, and high spectral resolution. For typical samples shown in the Figure 2, 1064 nm Raman spectra of acetaminophen, aspirin, and caffeine were acquired on powder material.

Figure 2: Raman spectra of acetaminophen, aspirin, and caffeine acquired with 1064 nm Raman microscope.
As an example, 1064 nm Raman imaging of Excedrin® Extra Strength Tablet, which has acetaminophen (250 mg), aspirin (250 mg), and caffeine (65 mg) as its APIs, was executed. As comparing the Raman features of the three major APIs, unique Raman features for each component were selected by markers with different colors to reconstruct the Raman image. As the color-coded Raman image in Figure 3c, the distributions of three APIs in the scanned region were highlighted: the red region was indicated as aspirin while the green and blue represented where acetaminophen and caffeine were located.

Figure 3: Color-coded Raman image of Excedrin® Extra Strength Tablet for discovering the spatial distribution of the major components - aspirin, acetaminophen, and caffeine (C). (A) indicates the selected Raman features of each chemical constituent for Raman imaging and (B) is the bright-view image.
BaySpec, Inc.
1101 McKay Drive, San Jose, CA 95131
tel. (408) 512-5928, fax (408) 512-5929
Website: www.bayspec.com


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














