Nondestructive Spectroscopic Techniques for Detection of Fungal and Mycotoxin Infections in Food Products: A Review
Fungal infections and mycotoxin contamination in food products pose a major threat to the world population. Mycotoxins contaminate approximately 25% of the world’s food products and cause severe health problems through the utilization of affected food products. The major mycotoxins in different foods are aflatoxins, ochratoxins, fumonisins, zearalenone, trichothecenes, and deoxynivalenol. Today, various conventional and nondestructive techniques are available for the detection of mycotoxins across multiple food products. Conventional methods are time-consuming, require chemical reagents, and include many laborious steps. Therefore, nondestructive techniques like near-infrared (NIR) spectroscopy, Fourier transform infrared (FT-IR) spectroscopy, hyperspectral imaging, and the electronic nose are a priority for online detection of fungal and mycotoxin problems in different food products. In this article, we discuss recent improvements and utilization of different nondestructive techniques for the early detection of fungal and mycotoxin infections in various food products.
Contamination of food and agricultural products by several types of toxigenic molds (fungi) is a severe and often overlooked problem. Regardless of years of study, scientists still have not discovered an effective way to resolve this challenging issue (1). Some of the fungal species like penicillium, alternaria, aspergillus, and fusarium can grow quickly on food products and food ingredients under favorable conditions and produce mycotoxins, which are harmful for humans and animals (2). The word mycotoxin is resulting from two words: “mukes,” denoting to “fungi” (Greek), and “toxicum,” denoted to “poison” (Latin). Mycotoxins are generally larger molecules that are significantly volatile (3).
Significant losses of harvested crops are due to chemical factors (residues of fungicides, pesticides, and herbicides), microbial factors (fungi and bacteria), and biological factors (different storage pests). These three factors are also responsible for discoloration, off-flavors, and declined nutritional value (4). The Food and Agricultural Organization of the United Nations stated that currently more than 25% of food production all over the world is affected by the fungal problem. Besides damaging the food products by discoloration, fungi can also release toxins and change the flavor of food, resulting in the food products acquiring a bad taste and odor (5).
Fungi are single-celled organisms that are heterotrophic. Nearly 7,000 fungal species are formally classified, but it is estimated that about 150,000 species exist (6,7). Fungi mostly colonize and produce mycotoxins during the preharvest (on crops in the field) or postharvest phases (during transport, storage, and further handling), which cause health risks for humans and animals (8,9). Even though these mycotoxins are present in very low quantities as parts per million (ppm) or parts per billion (ppb), severe or long contact with mycotoxins has been associated with liver cancer (10), immunosuppression in children (11,12) and, in some cases, chromic illness and death (13). If timely detection is possible and we are able to remove fungal-contaminated cereal or grains, we can ensure long-lasting storage, food safety, and seed quality (14). Normally, filamentous fungi (fungi imperfecti) that are modified to the terrestrial environment are generally known as mycotoxin producers. These fungi colonize and utilize solid substrates by penetrating deeply into their matrices with the help of enzymes to break down complex matrices into simple ones. In the maximum number of cases, the colonizing fungi create low molecular-weight compounds (with set poisonous properties), usually stated as “mycotoxins or secondary metabolites,” which are unsafe and have adverse health effects on human and animal growth and survival (15).
Human exposure to mycotoxin usually occurs by inhalation, ingestion, and dermal contact, and is mostly occurring unintentionally. In most cases, the problem starts in animals and human beings by eating infected food. Human interaction can be through cereals, fruits, and vegetables and indirectly through different animal produce such as meat, eggs, milk, and butter (16). Maximum numbers of mycotoxins are comparatively heat stable during the conventional food preparation temperature range (80–121 °C); therefore, very little (or none) degradation of mycotoxins occurs in the usual cooking circumstances, such as frying, heating, boiling, or pasteurizing (16). The contamination due to the presence of aflatoxin in food products is a serious problem all over the world. Many studies focused on aflatoxin contamination in food products have reported alarming statistics in different countries, particularly in tropical and subtropical regions like Asia and Africa (16).
Nondestructive analytical methods for the detection of fungal infections and mycotoxin contamination have become more popular and used regularly (17). The modern imaging acquisition and processing techniques have potential for the evaluation of agricultural and food products by nondestructive techniques (18). Such techniques include thermal imaging (19), X-ray microcomputed tomography (20), and the electronic nose (21), Fourier transform infrared photoacoustic spectroscopy (FT-IR PAS) (22), color imaging (23), hyper-spectral imaging (24), near-infrared (NIR) spectroscopy (25), and neutron tomography (26). These techniques have been studied for their detection of fungal and mycotoxin contamination in food products.
We describe in this article the current improvements in nondestructive measurements of fungal infections and mycotoxin contamination along with food safety testing in different food products like cereals, pulses, fruits, and vegetables.
Types of Mycotoxins
Aflatoxin
There are different types of aflatoxins present in food. Aflatoxin B1 is a familiar human carcinogen, and B1 is also considered a Group 1 carcinogen. The World Health Organization (WHO) also reported liver cancer or hepatocellular carcinoma (HCC), which is considered the third leading cause of death in the world (27). Therefore, the International Agency for Research on Cancer (IARC) has categorized aflatoxin B1 as a carcinogen for humans and animals years ago (28). Aflatoxin B1 causes contamination in agricultural products; as a result, many countries have instituted very strict limits for aflatoxin B1 in food and agricultural products. The European Commission (EU) has defined the maximum limits of aflatoxin B1 contamination in grains, beans, ground nuts, corn, cereals, spices, and rice as 5 mg/kg (29).
Citrinin
Citrinin is widely produced by penicillium (30) and causes nephrotoxic and hepatotoxic effects in different animal species. Citrinin had a role in the Bakin endemic nephropathy that was recorded and also reported inhibiting the protein, DNA, and RNA synthesis in porcine kidneys at the concentration level of 0.01 mM (31). Therefore, toxicologically, it is linked with a destructive synergistic effect, like DNA, damaging in renal cells (32). Citrinin also causes health and environmental issues in different types of agricultural products (33).
Ochratoxin A
Ochratoxin A is considered one of the most powerful mycotoxins that may be found in human food and in animal feed. It is also responsible for major contamination in staple foods and different types of commodities. Humans are directly or indirectly affected by ochratoxin A; it enters the food chain by contaminating the ingredients of food products consumed by humans. It also contaminates animal feed ingredients (34). Studies show that ochratoxin A is mainly caused by nephrotoxicity, genotoxicity, immunotoxicity, and neurotoxicity in the different type of animals (16).
Fumonisins
Fumonisins cause different problems in the human body. Fumonisin B1 stimulates the proliferation of regular human esophageal epithelial cells, increasing the protein appearance of cyclin D1 and reducing cyclin E, p21, and p27 (35). The most common present fumonisin analogs is fumonisin B1; it is categorized as a 2B cancer-causing agent for humans since it is harmful to them (27,36). Studies show that FB1, FB2, and FB3 are major contamination-causing fumonisins in cereals (16). Therefore, fumonisins have been found to be toxic to kidneys and liver; they have also been shown in vivo to be nephracarcinogenic in male rats and hepracarcenogenic in male rats and female mice (37).
Trichothecenes
Trichothecenes consist of more than 200 compounds, which are further classified into four subgroups (Types A, B, C, and D) based on their different functional groups. Therefore, a group of B has a ketone at position C-8, and the group of A has a ketone other than the position of C-8, group C has a second proxy group at C-7, C-8, C-9, or C-10, and group D has a macrocyclic rig between C4 and C5 (16).
Trichothecene affects ribosomal protein synthesis, which also causes a disturbance in the synthesis process of DNA and RNA (36–39). Trichothecene is also responsible for apoptosis and cytotoxicity (16). It is causing toxicity in animals, resulting in decreased appetite, so animal decreased intake of feed (anorexia) and exhibit vomiting (emesis) resulting in the reduction of animal growth and causing adverse effects on the heart, spleen, thymus, liver, and kidneys (40).
Detection of Fungal and Mycotoxins Contamination in Cereals
Fungal infection is a major cause of reduced product quality and market value of the cereal products all over the world. Penicillium, fusarium, and aspergillus are the main species of fungi that cause contamination problems in cereals and grains. Therefore, if the fungal problem is not identified during the early contamination levels, some species produce harmful mycotoxins (41). Major cereals of the world are rice, barley, wheat, maize, millet, oat, sorghum, and rye. A major problem related to cereals is that fungal infections belong to the genera fusarium, alternaria, aspergillus, and penicillium (12).
Studies show that different fungal problems in cereals, producing toxins like fusarium in wheat grains, is the cause of deoxynivalenol (DON), a prominent toxin (42). The contamination caused due to the presence of DON results in nausea, vomiting, and acute illness (43). The aflatoxin varieties B1, B2, G1, and G3, are well-known major causes of disease. Aflatoxin B1 contains the highest risk for causing toxicity and can also cause hepatitis in human beings (28). Aflatoxins act as nephrotoxins and also cause teratogenesis, and is found in many food products, such as wheat, rice, and maize (44). To reduce the health issues and economic losses, it is compulsory to monitor fungal problems properly and regularly during storage. On hard surfaces, fungal growth can easily be removed, but the different types of mycotoxins that grow in grains are not removed during most different processing steps. Therefore, timely recognition of fungal infections in cereals and grains is useful for controlling this problem (45).
Detection of Fungal and Mycotoxins Contamination in Fruits
Early detection of fungal problems in citrus fruits is useful in packing lines to minimize economic losses and protect food quality. Studies show that ultraviolet illumination is used to identify and remove rotten affected fruits manually. Early detection of rotten fruits is possible by the use of a hyperspectral imaging system, noting the rottenness problem caused by penicillium, is detectable by hyperspectral imaging in a closed area (46). It is problematic to detect different fungal ailments that lay in or on a small number of fruits; therefore, these fungal problems spread to adjacent objects such as healthy strawberry fruits in a storage container. The result is that you suffer losses in low quality and less revenue in the market (47). For example, a strawberry fruit, which has a short post-harvest lifespan, lasts less than five days due to physiological disorders, dryness, infections, and mechanical injuries produced by a wide range of bacteria, fungi, and viruses (48, 49). Apple and unpasteurized apple juice can be contaminated with Escherichia coli, which is considered a well-known problem related to food safety (45). Fusarium, rhizopus, and alternaria rot cause a major problem in tomato fruits at different levels. Fusarium rot is more damaging for ripe and red tomatoes than green ones (50,51).
Detection of Fungal and Mycotoxins Contamination in Vegetables
Leafy vegetables can also get contaminated, bringing about health-related concerns. Leafy vegetables are eaten fresh as a salad and can also be cooked. Studies show that E. coli on packaged spinach was evaluated by using hyperspectroscopic technique applied over the spectral range of 400 to 1,000 nm (45). Deterioration of vegetables is mostly caused by different types of fungal infections, such as penicillium digitatum (52,53). Damage of vegetables not only affects their sensory properties but also involves the risk of spreading infections to a complete growing patch and therefore leads to economic losses at a high level (46). Therefore, vegetables having defects that are not obvious are not detected and may seriously influence the vegetable quality and market value. Defective vegetables that can be easily identified are able to be removed by using nondestructive imaging techniques (54).
Detection of Fungal and Mycotoxins Contamination in Meats and Nuts
The meat industry also requires a nondestructive method for precisely detecting microorganisms that cause spoilage in meat products. FT-IR techniques are commonly used in the meat processing industry to determine biochemical changes occurring due to microbial spoilage of fresh beef placed at room temperature for 24 hours for study purpose (55). During storage, FT-IR images were taken after every hour and resulted in analysis of total viable counts (TVC) of the sample (56).
The different type of mycotoxins also pose a serious threat for the nuts during production, transportation, different processing practices, and even during storage. During the manual sorting of nuts, we cannot detect infectious and deteriorated nuts inside the shells. Studies show that we detect the inner quality of nuts without deshelling them by the use of NIR spectroscopy with the wavelength range of 2,200 to 2,500 nm (57). During the evaluation of pistachio nuts against the aflatoxin contamination and kernel necrotic spots, an X-ray line scanning system was successfully implemented (58). Ochratoxins (A, B, and C) are metabolites normally formed by the A. ochraceus and penicillium verrucosum (59). Ochratoxin A is a strong nephrotoxin and is often present in dry nuts (60,61).
Detection of Fungal and Mycotoxins Contamination in Seafood
Different types of seafood such as “sashimi” or “sushi” can be easily infected by pathogenic microorganisms and may be involved in foodborne diseases. Fillets of marine fish crustaceans, mollusks, different types of fish like roe, or other seafood products are mostly affected. Vibrio parahaemolyticus is primarily present in seafood whereas salmonella and staphylococcus can enter these food products during food processing, inappropriate handling practices, and poor storage conditions (62).
Detection of Fungal and Mycotoxins Contamination in Spices and Medicinal Herbs
A survey conducted in Spain showed that different types of mycotoxins, aflatoxin, zearalenone, ochratoxin A, fumonisin, and citrinin were found in 84 different types of medicinal herbs (63). A survey conducted in South Korea evaluated spices and treated spice foods against aflatoxin infection and found that aflatoxin B1 was found in 13.6% of spices and can be responsible for harmful spice contamination (64). Different type of mycotoxins like aflatoxin, ochratoxin, and fumonisins have been found in ready-to-eat products that were collected from different food markets and food centers (65–67). A survey was conducted in Jordan, collecting milk and meat from local markets and examining samples for aflatoxins. The survey showed that aflatoxins G1, G2, B1, and B2 were found in the meat samples (68).
Therefore, different traditional methods are available for the detection of mycotoxin and fungal contaminations using standard chemical analysis (69), the colony counting method (70), adenosine triphosphate (ATP) bioluminescence, polymerase chain reaction (PCR) method (71), and enzyme-linked immunosorbent assay (ELISA) (72), biosensors (73) and chromatographic methods (74). In these methods, more time is required for sample preparation and sometimes leads to sample destruction—so the need to develop rapid, simple, and accurate methods for the determination of mycotoxin infections and mycotoxin contamination is essential. Nondestructive techniques for the detection of fungal and mycotoxin infections need to become more popular and rapidly deployed (17).
Nondestructive Techniques for Detection of Fungal and Mycotoxin Infections
Near-Infrared (NIR) Reflectance Spectroscopy
Figure 1: Schematic diagram of the near-infrared (NIR) measurement process.
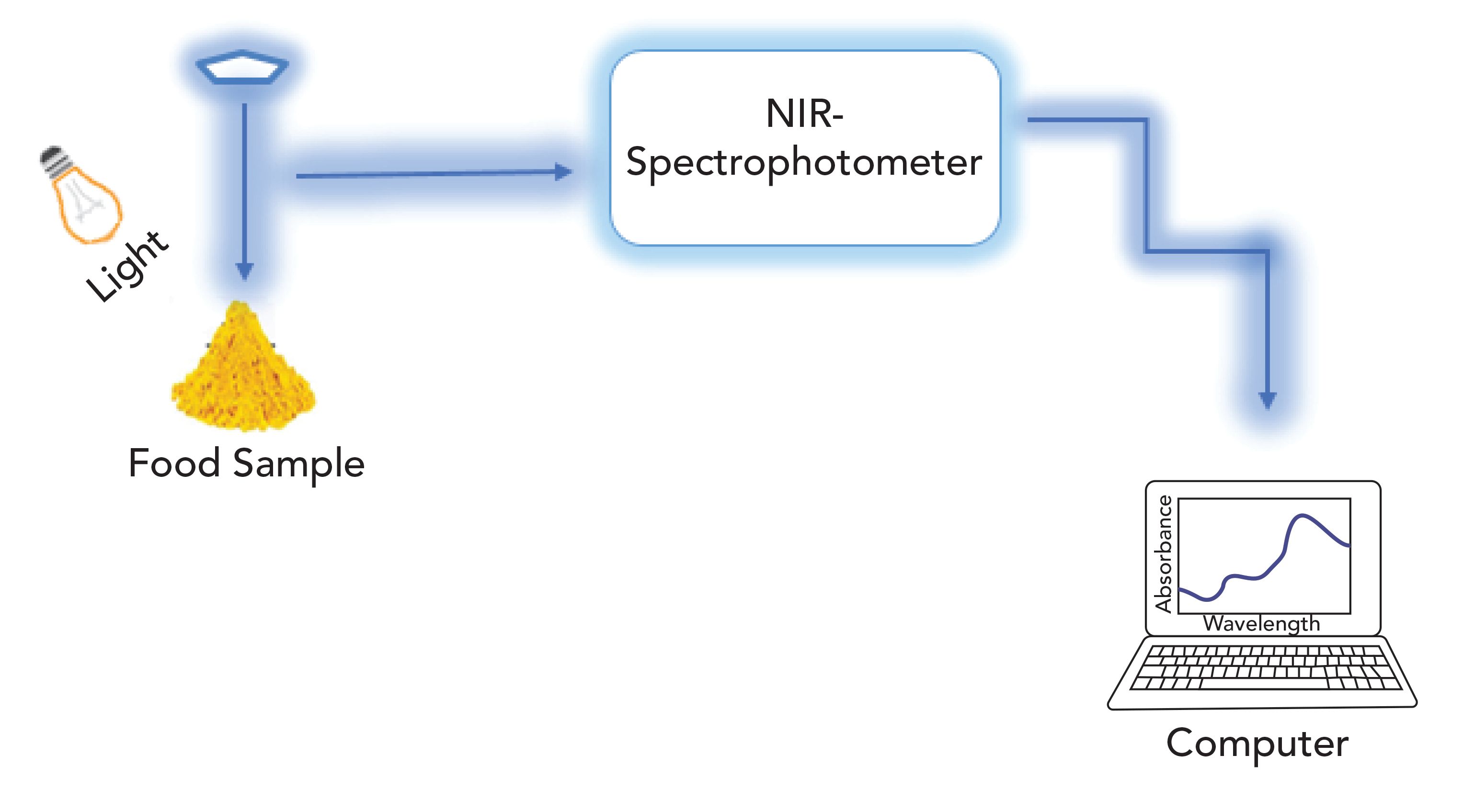
NIR spectroscopy is a nondestructive and rapid method now widely used for recognition of fungal and mycotoxin infection in food products. The electromagnetic spectrum in the range of 780 to 2500 nm (12,500 to 4000 cm-1) is normally used for NIR spectroscopy (Figure 1) (75). This technique also detects the components that have small concentrations. Many food industry researchers use NIR spectroscopy for measuring adulteration in food products due to its safety and the reliability of the data (76). NIR provides much more data associated with the structure by vibration behavior of combination bonds. The NIR region of the electromagnetic spectrum comprises the response of the molecular bonds O–H, C–H, C–O, and N–H. These bonds are associated with vibrational energy, which is measured using NIR frequencies (75).
NIR spectroscopy in the spectral range of 950 to 1650 nm is generally used for the determination of fungal infections or mycotoxin contamination in rice samples. Original and preprocessed absorbance spectra are used with partial least squares regression (PLS-R). The statistical calibration model provides prediction results with a correlation coefficient (r) of 0.668, and a standard error of prediction (SEP) of 28.87%, with a bias of -0.10% (77). NIR spectra vary substantially for the different food products; so differences occur between the measured and predicted composition of varying food products (78). The extraction information from the large data sets and the complexity of the spectra are the main concerns with this nondestructive practice (79).
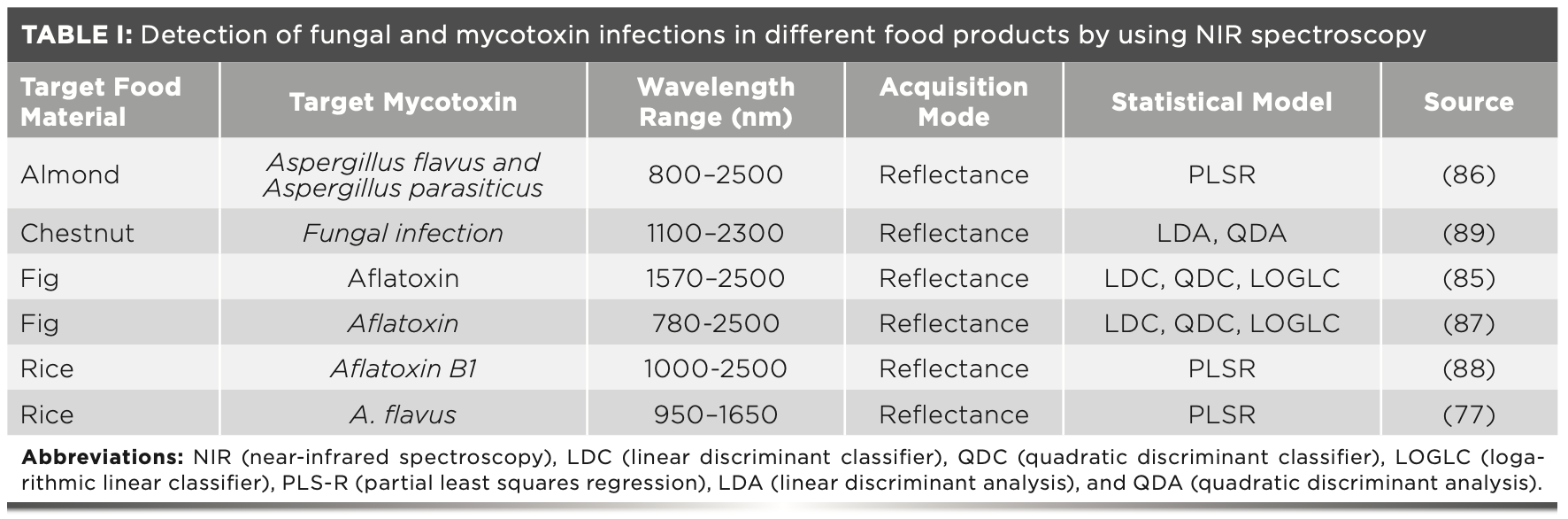
NIR spectroscopy techniques are also used for the estimation of fusarium head blight and identification of DON in wheat grains if the level is greater than 60 ppm of DON (80). NIR spectroscopy is also used for the detection of fumonisins in single maize seeds affected with Fusarium verticillioides. Maize seeds having a fumonisins level greater than 100 ppm were ranked as positive, whereas seeds with less than 10 ppm were ranked as negative (81). It is important to point out that a different types of mycotoxins being detected using NIR spectroscopy have not been visibly recognized. As a result, it is important that care must be taken when we develop calibration or classification models to measure or identify mycotoxins ppm levels, as several aspects contribute to the NIR method accuracy and sensitivity mentioned in Table I (82).
Compounds identified by the retention time evaluation of unknowns with those of standards and by standards adding or spiking (83). So, Hx and IMP are referred to standards; therefore, values of standards were calculated by external methods, using calibration curves of the peak area of compound versus concentration. The K1 value is obtained by the following equation 1 (84).
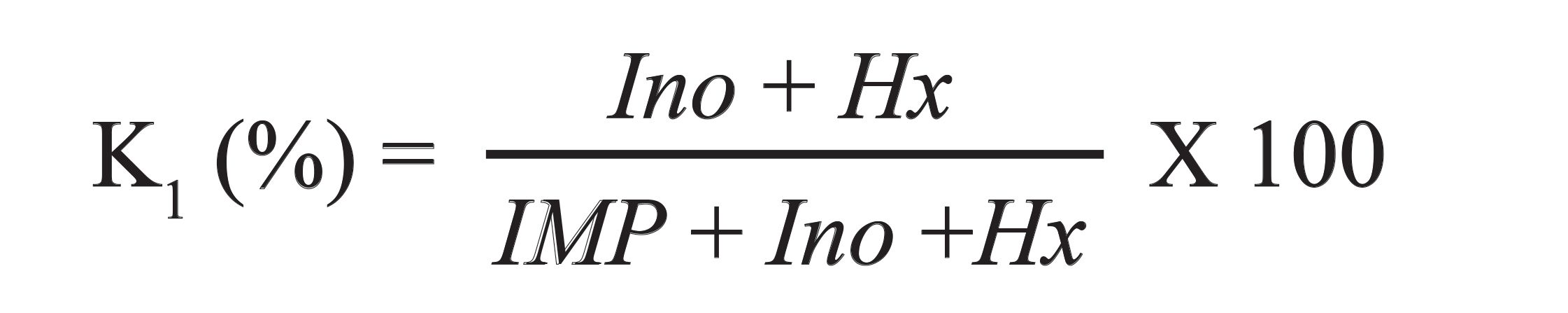
Fourier-Transform Infrared Mid-Infrared Spectroscopy (FT-IR MIRS)
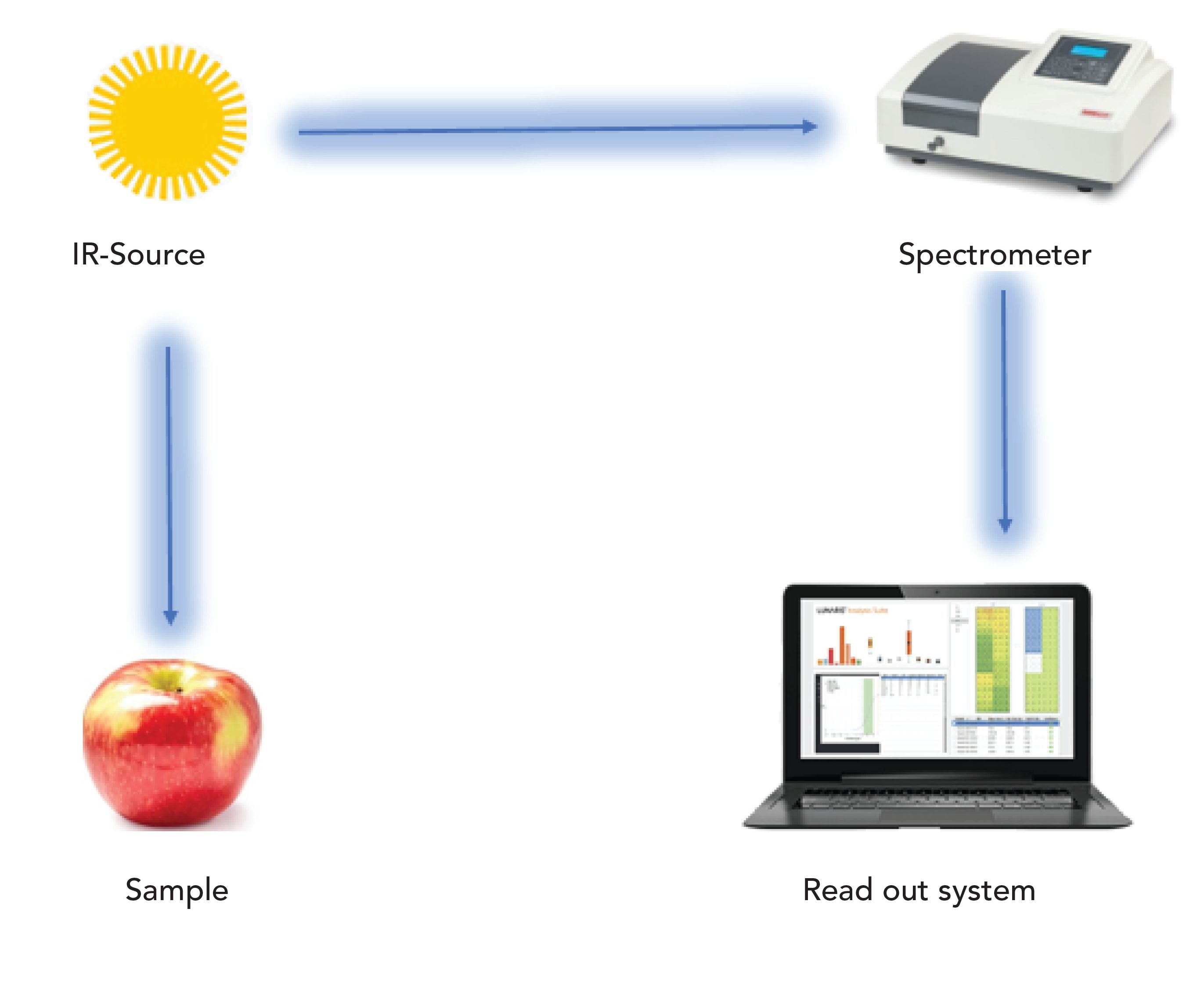
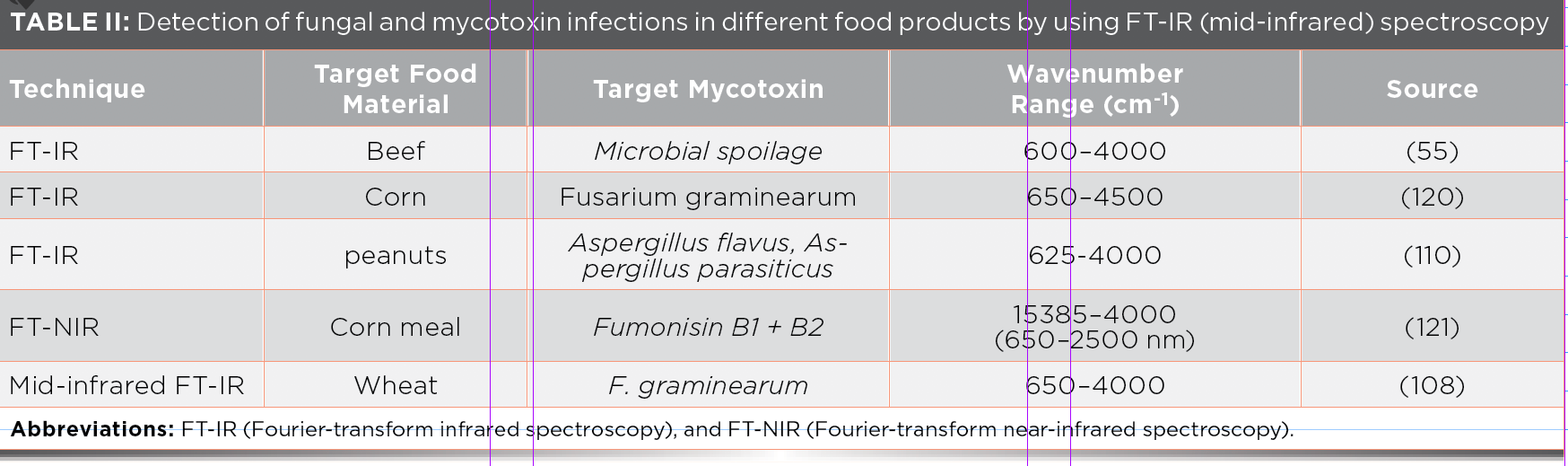
FT-IR is a nondestructive technique widely used in the world that measures the total infrared energy absorbed by the studied sample as seen in Figure 2 (90). FT-IR is an easily useable technique with little interference due to surface light scattering—it allows examination at sample depths from micrometers to 100 mm (91). Thus, the main benefit of FT-IR is the depth profiling ability for the nondestructive assessment of food materials (92). FT-IR sampling techniques are normally used for the examination of porous or powdered samples, catalysis, solids, and surface species (93,94). Since FT-IR–photoacoustic spectroscopy (FT-IR-PAS) applications for the food products are defined, it is commonly used in microorganism detection (95), food characterization (96), analysis of potato chips (92), analysis of coffee (97), and pea seeds analysis, mentioned in Table II (98).
Figure 2: Schematic design of the FT-IR measurement process.
In mid-infrared spectroscopy (MIRS), the spectral region is extended from 4,000 cm-1 to 400 cm-1 (2.5–25 μm), and it provides the compositional, chemical, and structural information on the constituent’s molecules for solid, liquid, and gas phase samples. As a result, MIRS has a wide application range in many fields (99,100), such as biotechnology (101,102), environmental analysis (103,104), material sciences (105), and medicine (106,107).
MIRS is an advantageous method for identifying microbial contamination and is also very effective at identifying mycotoxins and fungal infections in different agricultural products (108,109). MIRS shows better results in the reflectance technique for the identification of contaminated kernels (110), which is due to the high sensitivity of the MIR technique (111). MIRS has information from vibrations of all functional groups present in the food sample, especially C–O and C=O and not solely O–H, NH, and C–H. Thus, MIRS is considered better for this application than near-infrared spectroscopy (NIR) (112). Changes in the lipids, proteins, and carbohydrate contents can be easily identified and interpreted at the proper regions in the spectrum using MIRS (113).
Fourier transform infrared (FT-IR) spectroscopy is also a widely used technique in the food industry because it provides information at the molecular level and is also a rapid technique that does not require intensive sample preparation. This technique is recognized as being very useful for the study of different types of materials like fruit cuticles (114), leaves (115), wood (116), and antioxidants of herbs, grains, and fruits (117). FT-IR microscopy monitors the changes during the maturation of fruits like olive fruit to gain an understanding of the processes taking place and how they may be monitored using FT-IR (118). FT-IR has the potential to identify the maize kernels infected with fusarium moniliforme. When affected maize kernels with fusarium moniliforme were analyzed, the resultant spectrum is entirely different from the spectrum of uninfected corn seeds (22).
The relationship between the FT-IR absorbance spectra and analyte concentration is defined by Beer–Lambert’s law (119), in equation 2.
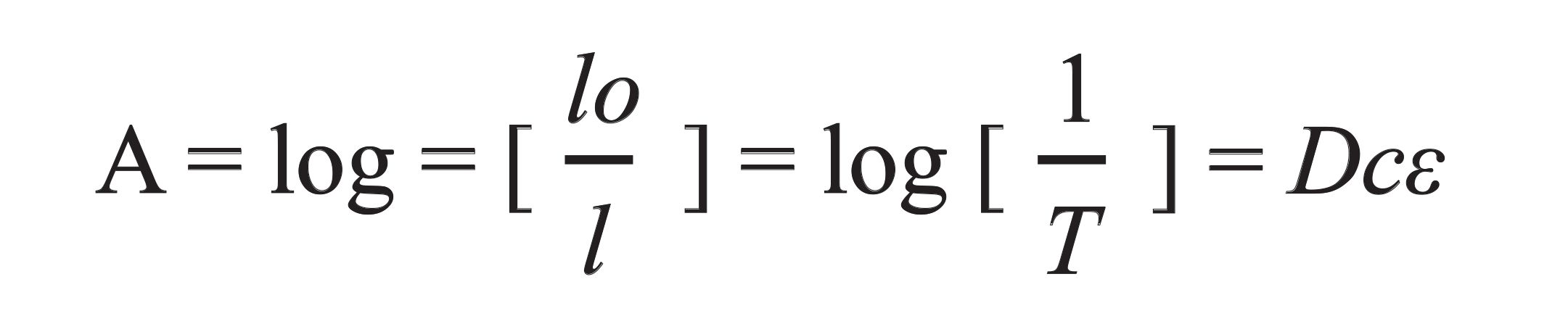
Note: I0 is intensity of incident light, I is the intensity of transmitted or reflected light, D is the absorption path length, and ε is the molar absorption coefficient.
NIR hyperspectral imaging
NIR hyperspectral is a nondestructive method that is combined with spectral and spatial information of the studied sample. The resulting data obtained from the NIR hyperspectral imaging is arranged into a hypercube, which is a three-way data cube that usually consists of hundreds of contiguous wavebands (wavelengths) for each spatial position of a sample under study (Figure 3) (79,122). In this method, the use of hyperspectral imaging, which allows the description of sample spatial (imaging constituent) and spectral (spectroscopic constituent) characteristics, is the fast and nondestructive technique now commonly used in the food industry (78). In NIR hyperspectral imaging, a hypercube is created, which can help in the analysis of intrinsic and extrinsic properties of the selected sample. Various studies are available for the use of NIR hyperspectral imaging for recognition of fungal contamination and pest problems in cereals and oilseeds (123).
Figure 3: Schematic diagrams of the hyperspectral imaging system.
Currently, hyperspectral imaging (HSI) is used for the rapid detection of many constituents. HSI takes images over a wide spatial range, but narrow spectral interval. Some multispectral imaging system offer spectral images with a broader spectral interval. HSI is a progressive inspection instrument for the identification of defects in different food products. Thanks to the line scanning process, it is now possible to use serial wavelengths to generate a 3D hypercube, which combines 2D images with 1D spectral information for every pixel present in the image patterns. HSI systems have advantages that provide both spectral and spatial information of the sample under study and is discussed in Table III (52).
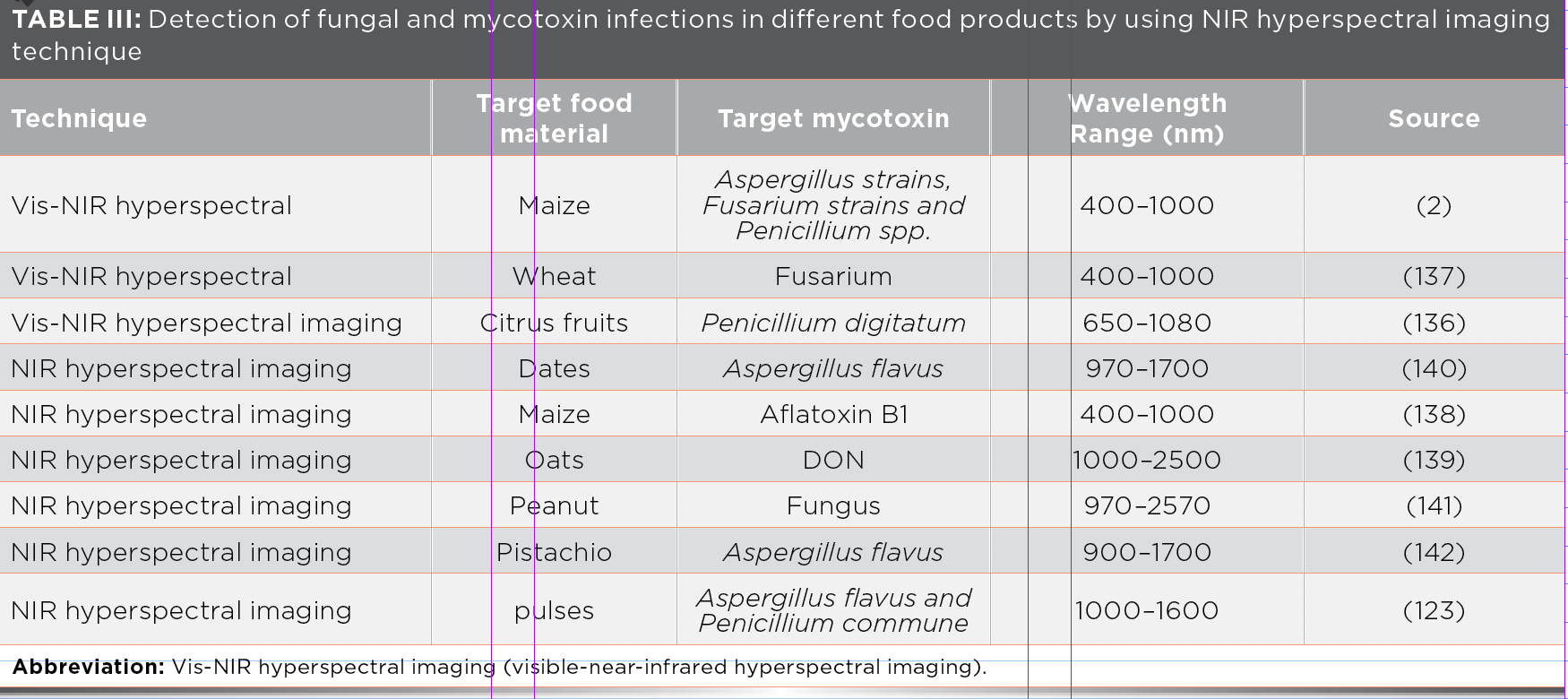
NIR hyperspectral imaging is also used to detect insect infestation in wheat seeds. The wheat seeds infested with Sitophilus oryzae (L.), Tribolium castaneum (Herbst), Rhyzopertha Dominica (F.), Tribolium castaneum (Herbst), and Cryptolestes ferruginous (Stephens) insects were scanned by using 100 to 1600 nm with an NIR hyperspectral imaging system. Ten histograms and six constituent statistical features (mean, median, minimum, maximum, standard deviation, and variance) were extracted from the images at 1101 nm and 1305 nm and also used in statistical discrimination classifiers (quadratic and linear). Using discrimination algorithms, classifiers differentiate the vigorously healthy and insect-affected wheat kernels with an accuracy of 85–100% (124).
NIR hyperspectral imaging techniques readily detect changes of whole maize grains infected with F. verticillioides. Hyperspectral images of infected and healthy kernels over a spectral range of 1000 to 2489 nm were applied at determined time intervals after infection started in kernels. Prominent peaks show the difference in spectra of the time intervals due to variations in the starch and protein at 1900 nm (starch associated) and 2136 nm (protein associated)—most probable cause is the reduction of these constituents as the fungus grows and spores germinate (125). More recently, NIR hyperspectral imaging has been used to analyze mycotoxins in cereal grains and evaluate aflatoxin B1 infections on the surface of maize kernels (126). In another study, a SWIR hyperspectral imaging system was applied to the identification of maize kernels where the aflatoxin infection was determined (127).
These studies show that NIR hyperspectral imaging techniques are demonstrating potential for determining insect infestation and mycotoxin infection in cereals and oilseeds. Therefore, the HSI detection tool has been useful for the detection of different contaminants and defects of agricultural materials, such as fruits (128), meat (129,130), grains (131–133); as well as mycotoxins in corn (125,134). In a hyperspectral imaging system, each image transforms into two kinds of images. One is the band difference image of successive spectral bands—these two kinds of images are defined as expressions 3 and 4.
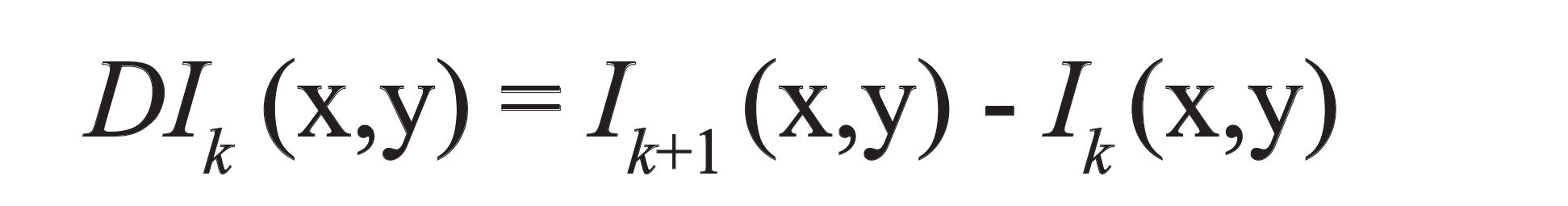
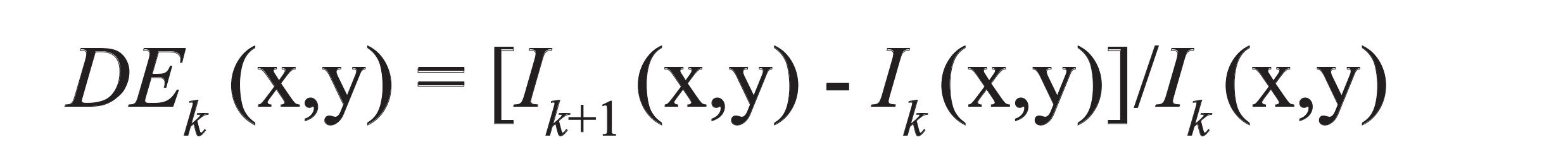
Here, Ik (x,y) links to pixel gray level at image point (x,y) for the kth spectral band for three types of images, indicated by (Ik, DIk, DEk) (135).
Electronic Nose
The electronic nose (e-nose) is also an innovative nondestructive technique widely used in food research applications. E-nose comprises an array of gas sensors along with diverse types of selectivity, a signal collection element, and a pattern-recognized system. It provides a high level of sensitivity for simple pretreatment procedures. The application of e-nose technology in food safety and beverages, quality control, and assessment, is quite easy. Solid and liquid samples are also analyzed and can detect headspace volatiles as seen in Figure 4 (143–147).
Figure 4: Schematic diagrams of the E-nose measurement system.
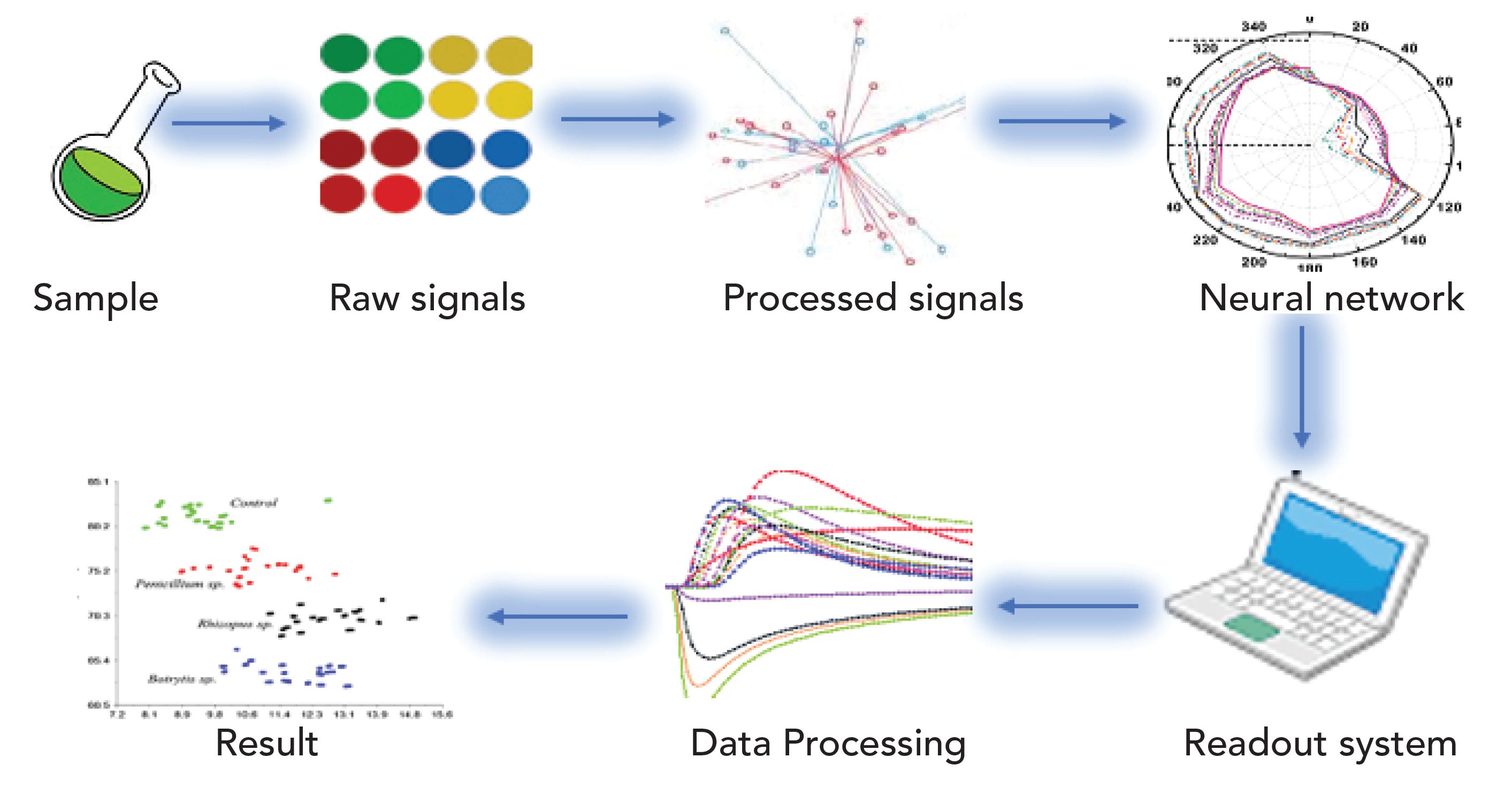
Therefore, an e-nose based on the nonselective sensors interrelate with volatile compounds that exist in the headspace of the sample that has a container. A signal is shown to the readout system, which is trained based on the training samples and calibration process, which leads to pattern recognition and analysis (148). Electronic nose data combined with the artificial neural network (ANN) was utilized for microbial level sorting of barley, rye, oats, and wheat samples (149).
In using e-nose, some investigations have been available to measure contamination by the foodborne microbes for different types of juices (145), blueberries (150), beef fillet spoilage (151), bread (152), and onions (153). In the case of strawberry fruit, the metabolites are produced by the yeast, including alcohols, esters, and acids, which alter the smell of the fruits as shown in Table IV (154). During storage, decay of strawberries can happen due to the fungal infection, and the contamination could spread to other unaffected fruits in the package container (155).
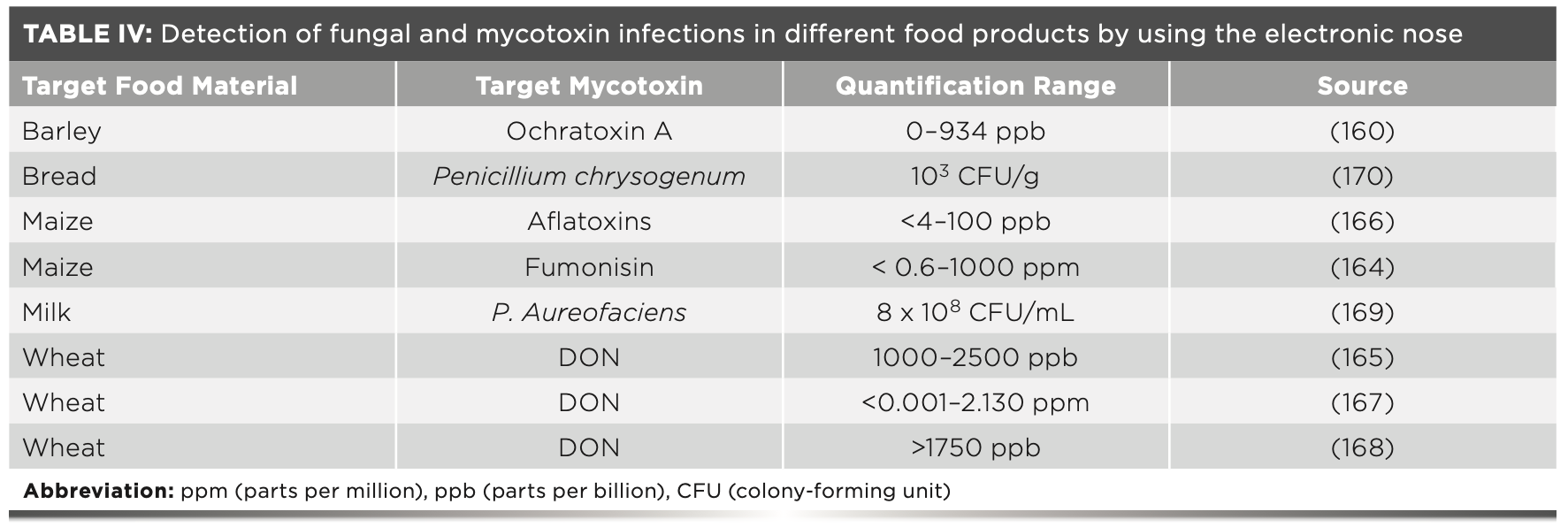
The unpleasant smell produced during spoilage is the result of a fungal:bacterial breakdown in the food products. Research shows that volatile chemicals produced by microorganisms could be detected and used to predict an early attack of microorganisms in different types of food products. Due to this, analysis of volatile compounds are increasing daily in the field of food research, while researchers work to improve the capability of odor sensing systems. Through the analysis of specific compounds responsible for the spoilage odors, detection through different analytical instruments provide a piece of useful information about food safety and the quality of the examined food products (156). Conventional analysis methods require more time, destroy test samples, and fail to provide timely results that are required by the fruit industry. Consequently, there is an important need for old tests to be replaced by the fastest, latest, sensitive, and nondestructive tests. It is helpful to monitor the infection of various food materials during storage and transportation, and by using spoilage analysis producers have the option to apply control measures (151).
The e-nose consists of numerous electronic gas sensors, which have the inherent selectivity and sensitivity to detect volatile compounds found in the headspace of the container. Many available articles relating to the practice of e-nose, and the analysis of different food products show positive results in the field of food quality (157). Although the complete chemical volatile profile is evaluated and analyzed, e-nose is used in combination with gas chromatography-mass spectrometry (GC–MS) (158–161). The e-nose detection technique used to detect fungal contamination is founded on the identification of specific volatile compounds that link with the growth of the particular type of microorganisms on different food products. E-nose only detects specified volatile compounds and shows the results using an attached computer (162).
In using e-nose, accuracy of classification was determined using the following equation 5 (163)
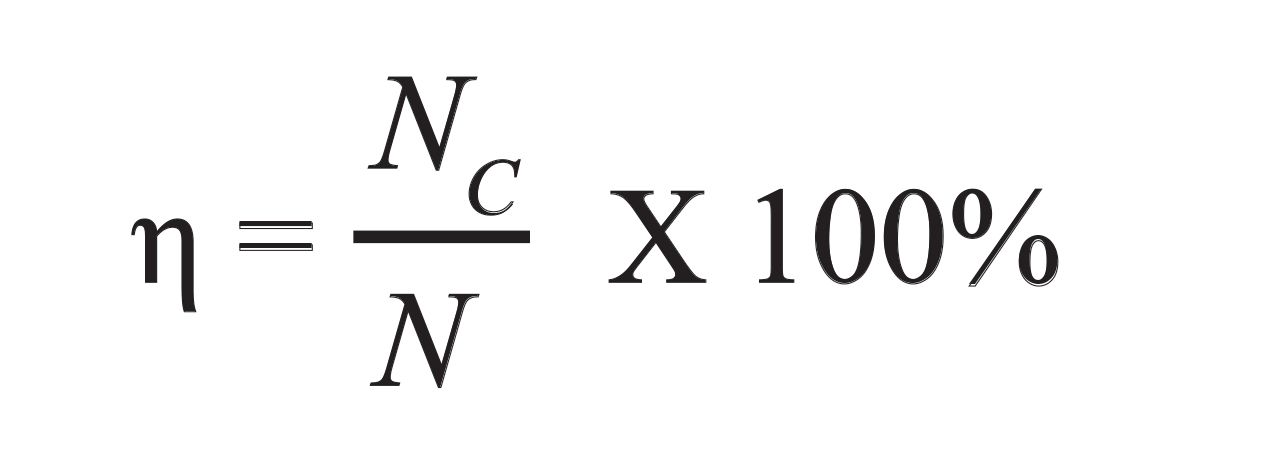
where η is the classification accuracy, Nc is the number of correctly classified samples, and N is the total number of tested samples.
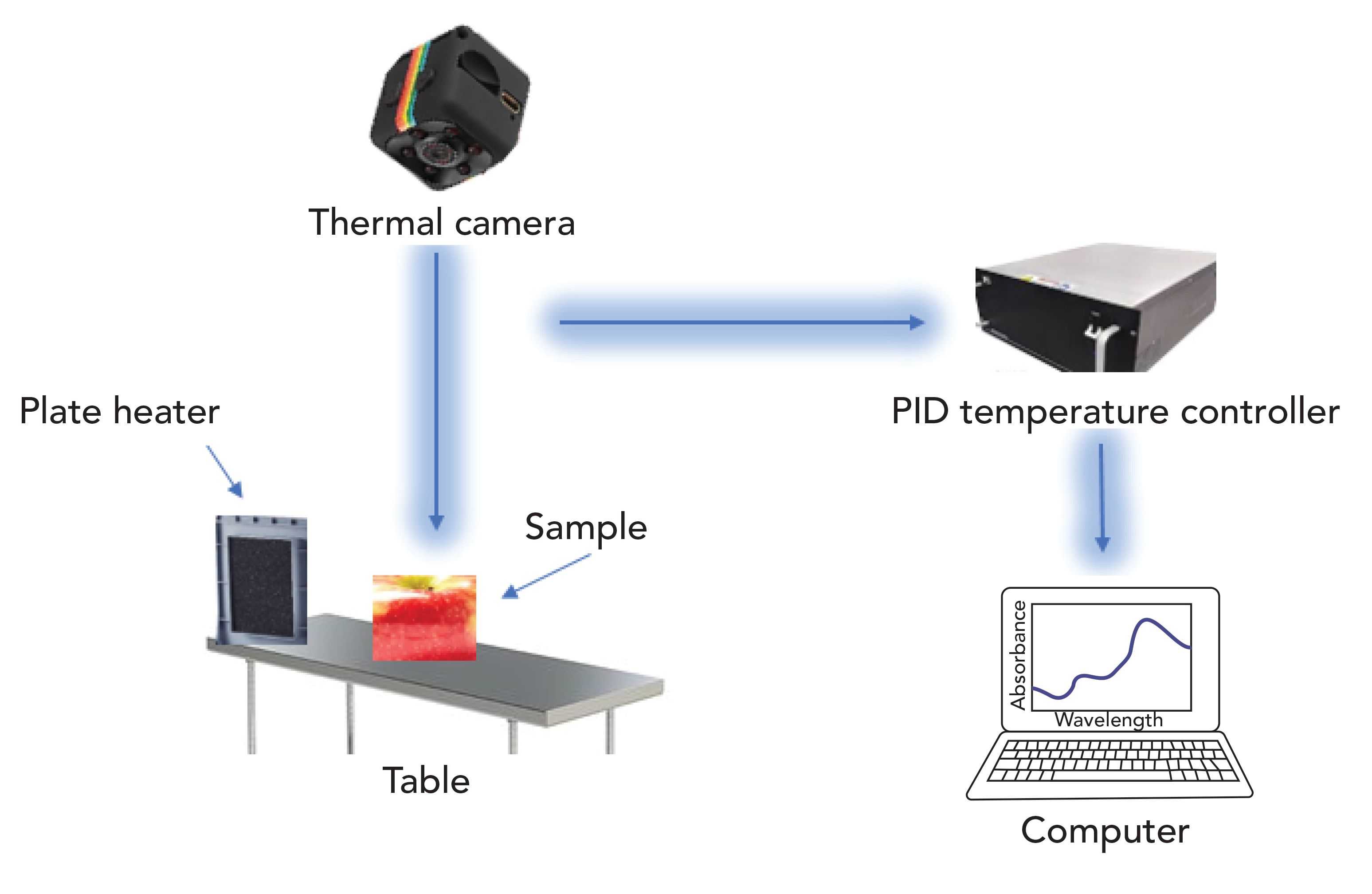
NIR Thermal Imaging
Fungal growth in food products, especially in cereals, causes some undesirable changes like off-flavor production, discoloration, and finally, quality loss, all of which decreases the market value of food products. Maximum mold growth occurs at the temperature range of 30–35 ̊C and with greater than 15% moisture content in samples (19). Note the following off-odors, 3-methyl, 1-butanol, 1-octanol, and 3-octane are the most common volatiles formed by the storage fungi. These off-odors are distinguished by numerous methods such as sensory analysis, gas chromatography, and mass spectrometry, which give accurate results, but these techniques required long sample preparation and testing time so there is a need to develop faster or more reliable techniques for early recognition of fungal contamination in stored cereals (162,171).
Near-infrared hyperspectral and soft x-ray imaging methods are used for the detection of Aspergillus niger van Tieghem, Penicillium spp, and Tieghem and the Aspergillus glaucus group of infections in the different types of stored cereals, especially in the wheat kernels mentioned in Table V (20,172). Therefore, the thermal imaging technique must have the ability to quickly and reliably detect infection in different agricultural products. This technique is built on temperature variations throughout the heating and cooling process (173). In the thermal imaging technique, the temperature radiation emission of the sample, which is unseen by the human eye, is transformed into a visible two-dimensional thermal image. The amount of radiations discharged by the sample increases with temperature, so thermal imaging permits us to see even small temperature differences (Figure 5). Today, thermal imaging is widely used in different industries like agriculture and electrical and civil engineering. In agriculture, this technique has been widely adopted to evaluate the quality of fruits and vegetables (174). It is also used to detect infections that occur due to microbial activities (175). Thermal imaging is also adopted in different grain handling practices like wheat class identification, and evaluation of insect infections and diseases (176).
Figure 5: Schematic diagram of the NIR thermal imaging system modified from reference (19).
The temperature of the surface of an object is estimated by the amount of infrared energy that is emitted from the body surface. The intensity of the distribution of energy emitted is based on Planck’s distribution law, expressed in equation 6, the temperature is expressed by (T, in K), and wavelength is expressed by (λ,T) (177).
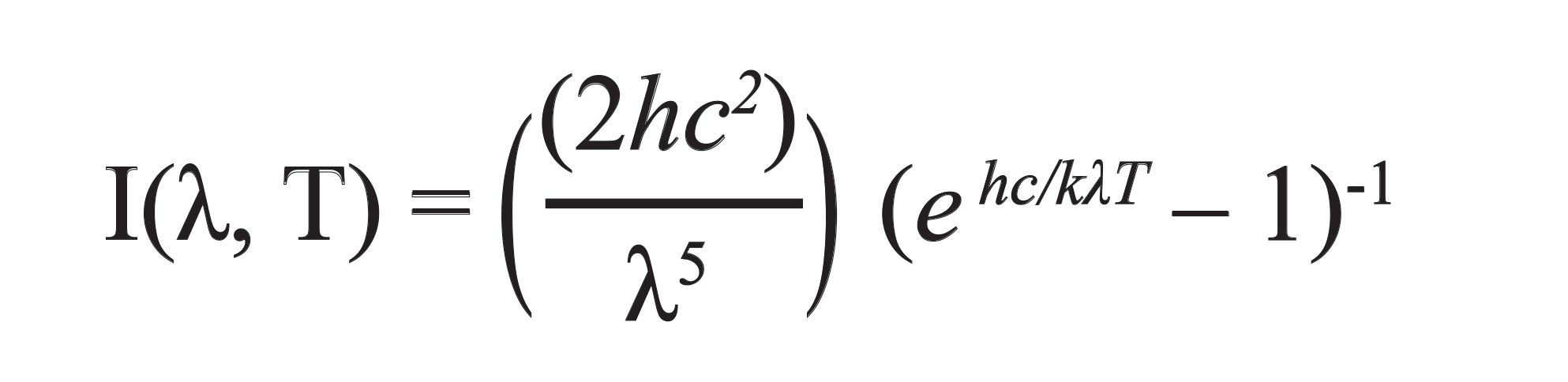
Surface-Enhanced Raman Scattering (SERS)
FT-Raman and surface-enhanced Raman scattering (SERS) is an inexpensive, fast, and nondestructive screening technique for the quantification and classification of aflatoxins present in different food products (178). Studies show that this Raman spectroscopic technique is a more interesting and powerful vibrational technique since it overcomes the constraints of infrared spectroscopy for determination and study of fumonisins in the different types of grains and oilseeds. Raman spectroscopic techniques show good response to a change in the polarizability of the symmetrical covalent bonds (such as S–S, C–C and C=C); however, infrared spectroscopy has shown to be the better technique for measuring dipole containing asymmetric covalent bonds (179).
Therefore, the Raman technique relies on the polarizability of a diverse set of bonds and not the dipoles of bonds like IR. SERS is also insensitive to water so it offers fewer and more distinct bands in food sample analysis. SERS is also used to study the structure of single components in complex grain matrices (180,181); and has the ability to detect a minor amount of harmful organic contaminants with more accuracy and reliability than other spectroscopic techniques (134,182,183). SERS also clarifies environmental pollutants and biomolecules that are adsorbed on metal surfaces.
Furthermore, SERS detects which molecular changes occur due to processing and is able to measure different factors associated with grain and food processing qualities (181,184). Having the capability to categorize grain kernels with mutant and recombined genes, it can be used to detect chemical modification of starches and proteins of different grains (181,185, 186). As a result, the analysis of spectral features provides detailed information about the sample at high resolution (187). SERS, used in conjunction with the density functional theory (DFT), gets more precise results and more information related to mycotoxins and calculations are more accurate than other rapid techniques.
Different studies have shown that Raman spectroscopic techniques are used in the combination of chemometric algorithms and multivariate statistical methods to study the different types of mycotoxins, such as fumonisins, deoxynivalenol (DON), and aflatoxins in barley, peanut, maize and wheat (178,187–190).
In Raman scattering, the presence of an electric field Ē induces a polarization in an atom and molecule described using the following equation 7, where α is the polarizability tensor.
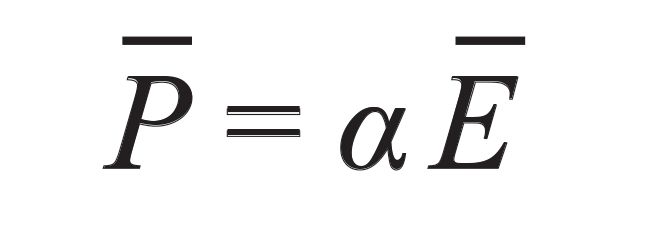
Also, the electric field of the incidence light is explained in the following way, whereas v0 is referred to as the frequency of the incident light (191).
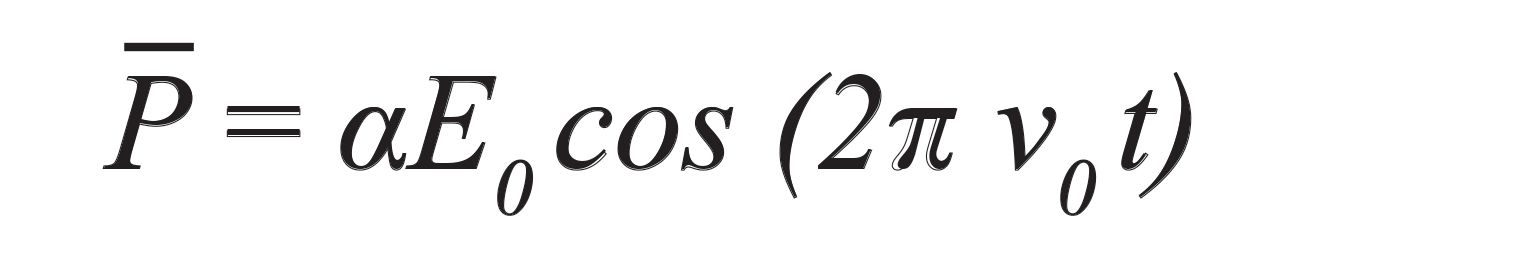
Future Trends and Needs
Mycotoxin problems increase daily around the world. They not only contaminate the food products and become the source of economic loss, but they also cause many diseases in humans and animals by eating contaminated food products. Studies show that many severe illnesses are caused by mycotoxin contamination, including but not limited to protein–calorie malnutrition, cancer, and HIV infection. Very few peoples know about the dangers of exposure of low-level mycotoxins over the long run, and the potentially severe health problems they cause so conventional methods are not the answer. These methods recognize mycotoxins in different food products, but they are time-consuming and contain tedious sample preparation processes. As a result, faster methods for detecting mycotoxins is needed, and spectroscopy is a possible alternative method that can detect mycotoxins more efficiently.
As a result, the expansion of nondestructive techniques is a great achievement for the detection of mycotoxins in different food products. Through these methods, we can easily detect mycotoxins in fruits, vegetables, meat, poultry, dry fruits, and spices by using different nondestructive techniques like NIR spectroscopy, FT-IR spectroscopy, hyperspectral imaging, E-nose, and others. In this article, we highlighted different spectroscopic methods that have wide potential use for determining mycotoxin problems in different food products. These techniques have the potential to determine massive amounts of contaminated food samples in a short period of time. Every method has particular advantages for the detection of mycotoxin problems in different food products. If spectroscopic data combined with chemometric techniques, then we can get qualitative and quantitative analyses of studied samples. Early detection of mycotoxins in food products reduces damage to fruits, vegetables, and cereals after harvesting. It also decreases the risk of spoilage and cross-contamination during processing and storage because deterioration increases due to the presence of mycotoxin-contaminated fruits, vegetables, and cereal products.
The methods reviewed in this article have the potential to replace the methods that are time-consuming and require tedious sample preparation. If these methods are commercially implemented, we will be able to control economic losses and protect human and animal populations from several diseases. However, nondestructive methods are not completely commercially implemented due to the high-cost of equipment, complex image processing, and the requirement for scientifically trained persons to run the nondestructive equipment. We also need to develop new strategies and protocols to compare the cost and benefits of different nondestructive methods for the detection of fungal problems, especially mycotoxins, aflatoxins, and deoxynivalenol in different food products.
We need to develop inexpensive methods for the evaluation of fungal and mycotoxins contamination in different food products at the field level because the detection of mycotoxins contamination at the field level is very difficult. We also need to develop methods for increasing the detection limits for small concentrations of mycotoxin levels in the different food products, reduction of required image size, and increased computational processing speed. With these improvements in detection and evaluation, nondestructive methods of detecting mycotoxins in food products will lead to enhanced international trading of food products, enhanced food safety efforts, and improved economic and public health conditions. In the future, we also need to develop rapid and reliable nondestructive methods for detecting fungal infections at harvesting farms and in the field. With these analysis methods we can easily separate contaminated food products from uncontaminated food products, and as a result, we save food products from cross-contamination during transportation and storage after the harvesting of fruits, vegetables, and cereals.
Conclusion
This article highlights the importance of nondestructive techniques for the determination of fungal infections and mycotoxins contamination of different food products. Before the development of nondestructive methods, conventional methods were widely used, but conventional methods require more time for determination and are also less accurate. Even with the increased use of technology, we still have not controlled this issue properly. Fungal infections and mycotoxins are still creating health issues in human beings and animals through contaminated food and feed. This issue also creates a negative economic impact as many food and feed materials are wasted every year because of fungal infections. Mycotoxins contaminate about 25% of the world’s production of food products. In different food products, we are able to separate mycotoxin-affected samples easily like potatoes, oranges, and strawberries, but in some food products, we cannot separate the affected samples, like in cereals. Early detection of fungal problems in food products is beneficial for applied control measures. If early detection is possible, we are enabled to separate the affected portion and save the remaining field, which is quite positive from an economic point of view. As this article highlights, we need to develop rapid, fast, reliable, and economical methods for early detection of fungal problems at the agricultural field level.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (31671844); the natural science foundation of Jiangsu Province (BK20160506, BE2016306); the National Key Research and Development Program of China (2016YFD0401104); International Science and Technology Cooperation Project of Jiangsu Province (BZ2016013) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
(1) G.P. Munkvold, Annu. Rev. Phytopathol. 41(1), 99–116 (2003).
(2) A. Del Fiore, et al., Int. J. Food Microbiol. 144(1), 64–71 (2010).
(3) H. Schiefer, Indoor air 90, 167–72 (1990).
(4) FAO, Methyl Bromide (F.A.A. Organization, Republic of Korea, 2005).
(5) FAO, Cereal Supply and Demand Brief: World Food Situation. Food and Agriculture Organization of the United Nations (accessed 17 April 14). http://www.fao.org/worldfoodsituation/csdb/en/. 2014.
(6) D.L. Hawksworth, Mycol. Res. 95(6), 641–655 (1991).
(7) D. Hawksworth, et al., Dictionary of the fungi. (1995).
(8) H.S. Hussein and J.M. Brasel, Toxicology 167(2), 101–134 (2001).
(9) N. Magan, V. Sanchis, and D. Aldred, Fungal biotechnology in agricultural, food and environmental applications. 311–323 (2004).
(10) J.H. Williams, et al., Am. J. Clin. Nutr. 80(5), 106–1122 (2004).
(11) Y. Gong, et al., Environ. Health Perspect. 112(13), 1334 (2004).
(12) Wagacha, J. and J. Muthomi, Int. J. Food Microbiol. 124(1), 1–12 (2008).
(13) L. Lewis, et al., Environ. Health Perspect. 113(12), 1763 (2005).
(14) M. Pasikatan and F. Dowell, Sorting systems based on optical methods for detecting and removing seeds infested internally by insects or fungi: A Review. (2001).
(15) R. Bhat, R.V. Rai, and A.A. Karim, Comprehensive reviews in food science and food safety. 9(1), 57–81 (2010).
(16) F. Bosco and C. Mollea, Mycotoxins in food, in Food Industrial Processes-Methods and Equipment. (2012).
(17) I. Orina, M. Manley, and P.J. Williams, Food Res. Int. 100, 74–86 (2017).
(18) P. Chen and Z. Sun, J. Agric. Eng. Res. 49, 85–98 (1991).
(19) V. Chelladurai, D. Jayas, and N. White, J. Stored Prod. Res. 46(3), 174–179 (2010).
(20) D. Narvankar, et al., Biosystems Eng. 103(1), 49–56 (2009).
(21) R. Paolesse, et al., Sensors and Actuators B: Chemical. 119(2), 425–430 (2006).
(22) R.V. Greene, et al., J. Agric. Food Chem. 40(7), 1144–1149 (1992).
(23) J. Tallada, et al., Transactions of the ASABE 54(3), 1151–1158 (2011).
(24) U. Siripatrawan and Y. Makino, Int. J. Food Microbiol. 199, 93–100 (2015).
(25) N. Berardo, et al., J. Agric. Food Chem. 53(21), 8128–8134 (2005).
(26) T. Cleveland IV, et al., J. Cereal Sci. 48(2), 517–525 (2008).
(27) C.P. Wild and Y.Y. Gong, Carcinogenesis 31(1), 71–82 (2009).
(28) IARC, International Agency for Research on Cancer, Geneva. 245 (1993a).
(29) H.A.T. Regulation, Commission Regulation (EC), J. Investig. Allergol Clin Immunol. 16, 136–137 (2006).
(30) H. Min and B.K. Cho, Biosyst. Eng. 40(1), 67–77 (2015).
(31) V. Aiko and A. Mehta, J. Biosci. 40(5), 943–954 (2015).
(32) Y. Wu, et al., Toxicology in Vitro 23(6), 1170–1178 (2009).
(33) Y.Z. Wang, X.L. Ju, and Y.G. Zhou, Food Microbiol. 22(1), 145–148 (2005).
(34) H.A. Clark and S.M. Snedeker, J. Toxicol, Part B: Critical Reviews 9(3), 265–296 (2006).
(35) S.K. Wang, et al., Experimental and Therapeutic Medicine 7(1), 55–60 (2014).
(36) M.E. Zain, J. Saudi Chem. Soc. 15(2), 129–144 (2011).
(37) E. Mazzoni, et al., Pest Management Science 67(4), 458–465 (2011).
(38) A. Desjardins and R. Proctor, Int. J. Food Microbiol. 119(1–2), 47–50 (2007).
(39) J.L. Richard, Int. J. Food Microbiol. 119(1–2), 3–10 (2007).
(40) P. Sobrova, et al., Interdisciplinary Toxicology 3(3), 94–99 (2010).
(41) G.S. Shephard, Food Additives and Contaminants 25(2), 146–151 (2008).
(42) S. Delwiche, Transactions of the ASAE. 46(3), 731 (2003).
(43) J.J. Pestka, Anim. Feed Sci. Technol. 137(3–4), 283–298 (2007).
(44) N. Arroyo-Manzanares, et al., Advances in Chemistry. (2014).
(45) M. Teena et al., Food Bioprocess Tech. 6(7), 1621–1634 (2013).
(46) J. Gómez-Sanchis, et al., J. Food Eng. 89(1), 80–86 (2008).
(47) J. Kovach, R. Petzoldt, and G.E. Harman, Biological Control 18(3), 235–242 (2000).
(48) E.K. Mo and C.K. Sung, Postharvest Biol Tec. 45(2), 234–239 (2007).
(49) B. Sallato, et al., Span J. Agric Res. 5(1), 67–78 (2007).
(50) F. Hahn, Biosyst. Eng. 81(3), 249–259 (2002).
(51) F. Hahn, I. Lopez, and G. Hernandez, Biosyst. Eng. 89(1), 93–99 (2004).
(52) D. Lorente, et al., Food Bioprocess Tech. 5(4), 1121–1142 (2012).
(53) J. Li, et al., Postharvest Biol. Tec. 112, 121–133 (2016).
(54) B. Zhang, et al., Food Anal. Methods. 8(8), 2075–2086 (2015).
(55) D.I. Ellis, D. Broadhurst, and R. Goodacre, Analytica Chimica Acta. 514(2), 193–201 (2004).
(56) Y. Peng, et al., J. Food Eng. 102(2), 163–169 (2011).
(57) F.R. De Mello and V.M. Scussel, J. Agric. Sci. 1(2), 3 (2009).
(58) S. Yanniotis, et al., Procedia Food Sci. 1, 379–384 (2011).
(59) E.E. Creppy, Toxicol. Lett. 127(1-3), 19–28 (2002).
(60) D. Höhler, Zeitschrift fur Ernahrungswissenschaft. 37(1), 2–12 (1998).
(61) E. Petzinger and A. Weidenbach, Livest. Prod. Sci. 76(3), 245–250 (2002).
(62) L. Gram and H.H. Huss, Int. J. Food Microbiol. 33(1), 121–137 (1996).
(63) L. Santos, et al., J. Sci. Food Agric. 89(10), 1802–1807 (2009).
(64) S.H. Cho, et al., Food Chem. 107(3), 1283–1288 (2008).
(65) Y. Sugita-Konishi, et al., J. Food Prot. 69(6), 1365–1370 (2006).
(66) B. Romagnoli, et al., Food Control 18(6), 697–701 (2007).
(67) M. Mushtaq, et al., Int. J. Mol. Sci. 13(7), 8324–8337 (2012).
(68) S.M. Herzallah, Food Chem. 114(3), 1141–1146 (2009).
(69) H. Lin and M. Cousin, J. Food Prot. 48(8), 671–678 (1985).
(70) H. Gourama and L.B. Bullerman, J. Food Prot. 58(12), 1389–1394 (1995).
(71) A.L. Boutigny, et al., Plant Pathol. 61(3), 522–531 (2012).
(72) P.G. Meirelles, et al., Food Agr. Immunol. 17(2), 79–89 (2006).
(73) B. van der Gaag, et al., Food Control 14(4), 251–254 (2003).
(74) V. Pereira, J. Fernandes, and S. Cunha, Trends Food Sci. Technol. 36(2), 96–136 (2014).
(75) H. Cen and Y. He, Trends Food Sci. Technol. 18(2), 72–83 (2007).
(76) M.M. Paradkar, S. Sivakesava, and J. Irudayaraj, J. Sci. Food Agric. 82(5), 497–504 (2002).
(77) C.D. Sirisomboon, R. Putthang, and P. Sirisomboon, Food Control 33(1), 207–214 (2013).
(78) A. Gowen, et al., Trends Food Sci. Technol. 18(12), 590–598 (2007).
(79) M. Manley, Chem. Soc. Rev. 43(24), 8200–8214 (2014).
(80) K. Peiris, et al., Cereal Chem. 87(6), 511–517 (2010).
(81) F.E. Dowell, et al., Cereal Chem. 79(2), 222–226 (2002).
(82) K. Norris, J. Near Infrared Spec. 17(4), 165–166 (2009).
(83) E.L. Johnson and R.L. Stevenson, Basic liquid chromatography. (1978).
(84) R. Grau, et al., Food Res. Int. 44(1), 331–337 (2011).
(85) A. Gunes, et al., “Detection of aflatoxin contaminated figs using Near-Infrared (NIR) reflectance spectroscopy in Electronics, Computer and Computation (ICECCO),” paper presented at the 2013 International Conference, IEEE, 2013.
(86) P.S. Liang, et al., Biosyst. Eng. 137, 64–72 (2015).
(87) E. Durmus, A. Gunes, and H. Kalkan, “Surface mold detection on figs using nir spectroscopy and it’s effect on aflatoxin level in Signal Processing and Communications,” paper presented at the 22nd Applications Conference (SIU), IEEE, 2014.
(88) Z. Qiang, et al., Int. J. Agr. Biol. Eng. 7(4), 127–133 (2014).
(89) R. Moscetti, et al., Postharvest Biol. Tec. 93, 83–90 (2014).
(90) J. Ryczkowski, Appl. Surf. Sci. 256(17), 5545–5550 (2010).
(91) J. McClelland, et al., Handbook of vibrational spectroscopy (John Wiley & Sons, Ltd, 2002).
(92) S. Sivakesava and J. Irudayaraj, J. Sci. Food Agric. 80(12), 1805–1810 (2000).
(93) J. Ryczkowski, Catal. Today 124(1–2), 11–20 (2007).
(94) J. Ryczkowski, Catal. Today 68(4), 263–381 (2001).
(95) J. Irudayaraj, H. Yang, and S. Sakhamuri, J. Mol. Struct. 606(1–3), 181–188 (2002).
(96) J. Irudayaraj, et al., J. Food Sci. 66(9), 1416–1421 (2001).
(97) F. Gordillo‐Delgado, et al., J. Sci. Food Agric. 92(11), 2316–2319 (2012).
(98) N.S. Letzelter, et al., J. Sci. Food Agric. 67(2), 239–245 (1995).
(99) M.J. Maltesen, et al., AAPS PharmSciTech 12(2), 627–636 (2011).
(100) N. Misra, C. Sullivan, and P. Cullen, Curr. Eng J. 2(1), 4–16 (2015).
(101) M. Kansiz, H. Billman-Jacobe, and D. McNaughton, Appl. Environ. Microbiol. 66(8), 3415–3420 (2000).
(102) M.C. Estevez, M. Alvarez, and L.M. Lechuga, Laser & Photonics Reviews 6(4), 463–487 (2012).
(103) N.K. Niazi, B. Singh, and B. Minasny, Int. J. Environ. Sci. Tec. 12(6), 1965–1974 (2015).
(104) C. Reidl-Leuthner, et al., Atmos. Environ. 112, 189-195 (2015).
(105) L. Fernández Carrasco, et al., Infrared spectroscopy in the analysis of building and construction materials (InTech, 2012).
(106) R. Pandey, et al., TracTrends Anal. Chem. 64, 100–108 (2015).
(107) S. Hou, et al., Talanta 142, 110–119 (2015).
(108) B. Abramovic, et al., Acta Chimica Slovenica. 54(4), 859 (2007).
(109) C.B. Fígoli, et al., Int. J. Food Microbiol. 244, 36–42 (2017).
(110) H. Kaya-Celiker, et al., Food Control 44, 64–71 (2014).
(111) R. Bauer, et al., FTIR spectroscopy for grape and wine analysis (ACS Publications, 2008).
(112) M. Hossain and T. Goto, World Mycotoxin J. 7(4), 507–515 (2014).
(113) S.H. Gordon,et al., Biotechnol. Adv. 11(3), 665–675 (1993).
(114) E.J. Johnson, et al., Commun. Soil Sci. Plan. 38(17–18), 2461–2478 (2007).
(115) B.R. da Luz, New Phytol. 172(2), 305–318 (2006).
(116) A.K. Mooreand N.L. Owen, Appl. Spectrosc. Rev. 36(1), 65–86 (2001).
(117) D. Cozzolino, Antioxidants 4(3), 482–497 (2015).
(118) M. López-Sánchez, M.A.J. Ayora-Cañada, and A. Molina-Díaz, J. Agric. Food Chem. 58(1), 82–87 (2009).
(119) J. Haas and B. Mizaikoff, Annu. Rev. Anal. Chem. 9(1), 45–68 (2016).
(120) G. Kos, H. Lohninger, and R. Krska, Anal. Chem. 75(5), 1211–1217 (2003).
(121) B. Gaspardo, et al., Food Chem. 135(3), 1608–1612 (2012).
(122) Q. Chen, et al., TracTrends Anal. Chem. 52, 261–274 (2013).
(123) K. Karuppiah, et al., J. Stored Prod. Res. 65, 13–18 (2016).
(124) C.B. Singh, et al., Int. J. Food Prop. 15(1), 11–24 (2012).
(125) P.J. Williams, et al., J. Cereal Sci. 55(3), 272–278 (2012).
(126) W. Wang, et al., Food Control 42, 78–86 (2014).
(127) L.M. Kandpal, et al., Food Control 51, 171–176 (2015).
(128) G. ElMasry, et al., J. Food Eng. 81(1), 98–107 (2007).
(129) J. Qiao, et al., J. Food Eng. 83(1), 10–16 (2007).
(130) M. Kamruzzaman, et al., Analytica Chimica Acta. 714, 57–67 (2012).
(131) G. Firrao, et al., J. Cereal Sci. 52(2), 327–330 (2010).
(132) H. Kalkan, et al., Comput. Electron. Agric. 77(1), 28–34 (2011).
(133) J.M. Prats-Montalbán, A. De Juan, and A. Ferrer, Chemometr. Intell. Lab. 107(1), 1–23 (2011).
(134) R.P. Cogdill, et al., T. ASAE. 47(1), 311 (2004).
(135) H. Zhongzhi and D. Limiao, Comput. Electron. Agric. 153, 248–255 (2018).
(136) J. Li, et al., Comput. Electron. Agric. 127, 582–592 (2016).
(137) E. Bauriegel, et al., Comput. Electron. Agric. 75(2), 304–312 (2011).
(138) W. Wang, et al., J. Food Sci. 80(1), M116–M122 (2015).
(139) S. Tekle, et al., Cereal Chem. 92(1), 73–80 (2015).
(140) M. Teena, et al., J. Stored Prod. Res. 59, 306–313 (2014).
(141) J. Jiang, X. Qiao, and R. He, J. Food Eng. 169, 284–290 (2016).
(142) K. Kheiralipour, et al., Qual. Assur. Saf. Crop. 8(1), 129–135 (2016).
(143) S. Balasubramanian, et al., Food Control 19(3), 236–246 (2008).
(144) I. Concina, et al., Food Control 20(10), 873–880 (2009).
(145) E. Gobbi, et al., Food Control 21(10), 1374–1382 (2010).
(146) P. Liu and K. Tu, Food Control 23(1), 177–183 (2012).
(147) T. Rajamäki, et al., Food Control 17(1), 5–13 (2006).
(148) E.A. Baldwin, et al., Sensors 11(5), 4744–4766 (2011).
(149) A. Jonsson, et al., Int. J. Food Microbiol. 35(2), 187–193 (1997).
(150) C. Li, et al., Postharvest Biol. Tec. 55(3), 144–149 (2010).
(151) O.S. Papadopoulou, et al., Food Res. Int. 50(1), 241–249 (2013).
(152) R. Needham, et al., Sens. Actuators B Chem. 106(1), 20–23 (2005).
(153) C. Li, N.E. Schmidt, and R. Gitaitis, LWT. 44(4), 1019–1025 (2011).
(154) P. Ragaert, et al., Food Microbiol. 23(2), 154–161 (2006).
(155) Y. Zhao, et al., Afr. J. Biotechnol. 10(47), 9623–9630 (2011).
(156) T. Dymerski, T. Chmiel, and W. Wardencki, Rev. Sci. Instrum. 82(11), 101–111 (2011).
(157) M. Peris and L. Escuder-Gilabert, Analytica Chimica Acta. 638(1), 1–15 (2009).
(158) S. Esposto, et al., Developments in Food Science (Elsevier, Amsterdam, 2006), pp. 315–318.
(159) N.L. De Lerma, et al., Food Chem. 130(2), 447–452 (2012).
(160) J. Olsson, et al., Int. J. Food Microbiol. 72(3), 203–214 (2002).
(161) S. Pastorelli, et al., Food Addit. Contam. 24(11), 1219–1225 (2007).
(162) N. Magan and P. Evans, J. Stored Prod. Res. 36(4), 319–340 (2000).
(163) J. Gębicki and B. Szulczyński, Measurement 116, 307–313 (2018).
(164) E. Gobbi, et al., Food Res. Int. 44(4), 992–999 (2011).
(165) V. Lippolis, et al., Food Control. 37, 263–271 (2014).
(166) F. Cheli, et al., Biotechnologie, Agronomie, Société et Environnement. 13(s), 39–43 (2009).
(167) G. Tognon, et al., Vet. Res. Commun. 29(2), 391–393 (2005).
(168) A. Campagnoli, et al., Sensors. 11(5), 4899–4916 (2011).
(169) N. Magan, A. Pavlou, and I. Chrysanthakis, Sens. Actuators B Chem. 72(1), 28–34 (2001).
(170) G. Keshri, P. Voysey, and N. Magan, J. Appl. Microbiol. 92(1), 165–172 (2002).
(171) J. Schnürer, J. Olsson, and T. Börjesson, Fungal Genet. Biol. 27(2-3), 209–217 (1999).
(172) C. Singh, et al., T. ASABE. 50(6), 2171–2176 (2007).
(173) M. Annamalai, Thermal imaging for potential use in cereals and oilseeds handling (2007).
(174) J. Varith et al., Innov. Food Sci. Emerg. Technol. 4(2), 211–218 (2003).
(175) H.J. Hellebrand, H. Beuche, and M. Linke, Thermal Imaging, in Physical Methods in Agriculture (Springer, 411–427, 2002).
(176) R. Vadivambal, et al., “Temperature distribution studies in microwave heated grains,” paper presented at the CSBE/ASABE North-Central Intersectional Conference. Fargo, North Dakota, 2007.
(177) A. Gowen, et al., Trends Food Sci. Technol. 21(4), 190–200 (2010).
(178) K.M. Lee, et al., Int. J. Regulat. Sci. 1(1), 1–14 (2013).
(179) W. Smith and G. Dent, Introduction, Basic Theory, and Principles and Resonance Raman Scattering. Modern Raman Spectroscopy (John Wiley & Sons, Ltd., England, 2005).
(180) D. Himmelsbach, et al., Cereal Chem. 78(4), 488–492 (2001).
(181) C.Y. Ma and D. Lee Phillips, Cereal Chem. 79(2), 171–177 (2002).
(182) C. Zhu, et al., J. Hazard. Mater. 211, 389–395 (2012).
(183) G. Liu, et al., J. Hazard. Mater. 248, 435–441 (2013).
(184) A.M. Herrero, et al., Food Chemistry 109(1), 25–32 (2008).
(185) M. Sohn, D.S. Himmelsbach, and F.E. Barton, Cereal Chem. 81(4), 429–433 (2004).
(186) N. Wellner, et al., Starch‐Stärke 63(3), 128–138 (2011).
(187) A.E. Grow, et al., J. Microbiol. Methods. 53(2), 221–233 (2003).
(188) X. Wu, et al., Analyst. 137(18), 4226–4234 (2012).
(189) R.S. Golightly, W.E. Doering, and M.J. Natan, Surface-enhanced Raman Spectroscopy and Homeland Security: A Perfect Match? (ACS Publications, 2009).
(190) Y. Liu, S. Delwiche, and Y. Dong, Food Addit. Contam. 26(10), 1396–1401 (2009).
(191) L.F. Maia, et al., Studies in Natural Products Chemistry (Elsevier, Amsterdam, 2014), pp. 313–349.
(192) Manickavasagan, A., D. Jayas, and N. White, J. Stored Prod. Res. 44(2), 186–192 (2008).
(193) K.M. Lee, et al., J. Agric. Food Chem. 62(19), 4466–4474 (2014).
(194) K.M. Lee and T.J. Herrman, Food Bioprocess Tech. 9(4), 588–603 (2016).
Muhammad Bilal, Zou Xiaobo, Muhammad Arslan, Haroon Elrasheid Tahir, Zhihua Li, and Jiyong Shi are with the School of Food & Biological Engineering at Jiangsu University, in Zhenjiang, in The People’s Republic of China. Muhammad Usman is with the Beijing Advance Innovation Center for Food Nutrition and Human Health, at Beijing Technology and Business University, in Beijing, in The People’s Republic of China. Direct correspondence to: zou_xiaobo@ujs.edu.cn

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.