Quantification of Active Pharmaceutical Ingredients on Metal Surfaces Using a Mid-IR Grazing-Angle Fiber Optics Probe — An In-Situ Cleaning Verification Process
The authors describe an in-situ cleaning verification technique that promises to be faster and more efficient than traditional methods, which use swab sampling followed by HPLC analysis. The technique is based upon FT-IR spectroscopy in the middle-infrared (mid-IR) range using reflection at a grazing angle.
Cleaning processes for pharmaceutical manufacturing equipment are inspected closely, because inadequate cleaning procedures can result in adulterated or contaminated products. Thus, the need to verify cleaning between manufacturing runs presents a special challenge. For cleaning verification, high performance liquid chromatography (HPLC) often is used to evaluate samples collected from manufacturing equipment. The cleaning verification process involves swabbing the surface and extracting the sample from the swab, followed by analysis using an HPLC system. Most laboratories use an HPLC system with an autosampler that allows sample analysis to take place unattended. Therefore, cleaning the pharmaceutical equipment, collecting and preparing samples for HPLC analysis, acquiring data, and processing can result in up to two days of lost production time. Other drawbacks to using HLPC methods for cleaning verification include incomplete analyte recovery from the surface and cross contamination resulting from handling and treatment of samples between swab collection and subsequent analytical work. Therefore, an ideal verification method for cleaning procedures would be a fast, automated, in-situ, multicomponent analysis of the entire surface. With such an approach, errors and inadequacies associated with surface sampling procedures can be eliminated and result in time and money savings.
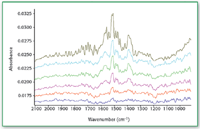
Figure 1. Mid-IR spectra of compound 1 at different levels (bottom to top: 0.05, 0.17, 0.47, 0.78, 0.86, and 1.64 üg/cm2) on metal coupons.
Recently, it was reported that in-situ spectroscopic techniques that employ middle-infrared (mid-IR) fiber optics and spectral imaging, for example, potentially could be used for the detection of small amounts of analytes from the surfaces of the equipment during the cleaning verification process (1â3). Thus, it is worthwhile to explore the feasibility of using mid-IR fiber optics for in-situ, noninvasive, accurate, and inexpensive cleaning verification. In the present study, the utility of such techniques in the submicrogram range is explored for the benefit of pharmaceutical manufacturing industries.
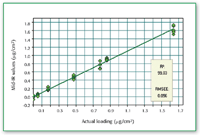
Figure 2. Calibration curve for compound 1.
Experimental
Grazing-angle spectra were obtained using a Remspec (Charlton, MA) SpotView mid-IR grazing-angle probe attached to a Bruker Optics (Billerica, MA) spectrometer with a Remspec external mercuryâcadmiumâtelluride (MCT) detector. The specially configured probe head illuminates a large spot on the sample surface (approximately 4.5 cm2) and maximizes the distance traveled through the surface layer by the IR beam before it returns to the detector. Spectra were collected across the range of 900â4000 wavenumbers (cm-1), and a clean metal coupon (a flat stainless steel polished metal surface of area 4 Ã 4 in.) was used to collect a background spectrum. Sample spectra were recorded at 6 cm-1 resolution using 100 scans with an approximate collection time of 0.5 min/spectrum). Opus 5.0 software from Bruker Optics was used for data acquisition.
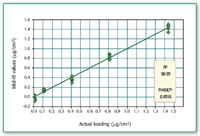
Figure 3. Comparison of predicted amount (using mid-IR) of compound 1 loaded on metal coupon. The actual amount loaded was determined by HPLC.
A calibration was built using the Quant 2 (Bruker Optics) package, an add-on to the Opus spectroscopy software. Quant 2 uses partial least square regression (PLS1). The calibration line generated was used to determine unknown amounts of the active pharmaceutical ingredient (API) loaded on the metal coupon. The samples also were prepared using a "smear" method. In the smear method, a known amount (0.1 mL) of a known solution of the target substance in methanol is dispensed onto a precleaned metal coupon. The solution then is "smeared" evenly across the surface and allowed to air dry. Alternatively, a solution of the target substance can be air-sprayed onto a pre-cleaned metal surface.
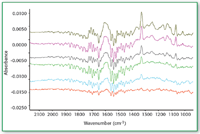
Figure 4. Mid-IR spectra of compound A at different levels (bottom to top: 0.07, 0.22, 0.32, 0.54, 0.79, and 1.12 üg/cm2) on metal coupons.
The coupons were dried, and sets of probe spectra were collected from at least six different locations on the metal coupon. The coupons were thoroughly washed, the washings analyzed by HPLC, and the "true" total surface concentration calculated. Reversed-phase HPLC methods developed in-house were used to determine the amount of APIs recovered using external standard. In the present study, three neat APIs (compounds 1, A, and AE) as well as a mixture of API (compound A) and excipients (Avicel, starch, lactose, magnesium stearate, and cabosil) were analyzed. A pure API stock solution in HPLC-grade methanol was prepared and then diluted to the microgram-per- milliliter range. The range of concentrations of these analytes used in the calibration was 0.05â1.5 μg/cm2. For each compound evaluated, a calibration equation was generated and the concentration of few unknown samples was determined using the calibration to validate the calibration process. The amount of API determined using the spectrometric calibration was compared with that obtained from HPLC analysis.
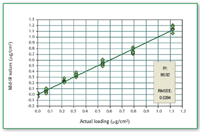
Figure 5. Calibration curve for compound A as neat analyte.
Results and Discussion
In the Fourier transform-infrared experiment, the signal amount acquired from a sample depends upon both the area illuminated by the IR beam and the path length traveled by the mid-IR radiation through the sample. The absorptivity of the samples for the concentration range used appears to be insignificant as the sample was deposited as a thin layer on metal coupon, as indicated by linear calibration plots. The grazing-angle head uses carefully aligned mirrors to deliver the mid-IR beam to the sample surface at the grazing angle (approximately 80° from normal) (4) to collect the reflected beam, and to return it to a mid-IR detector (in this case, an MCT detector). The signal is delivered from the spectrometer to the head and returned to the MCT detector by IR-transmitting fiber optic cables (measuring approximately 2-m long). The specially configured head illuminates a large spot on the sample surface (about 4.5 cm2) and maximizes the distance traveled through the surface layer by the IR beam before it returns to the detector. That combination of factors increases the sensitivity and signal-to-noise performance of the probe by several fold when compared with conventional reflectance methods using a mid-IR beam normal to the sample surface.
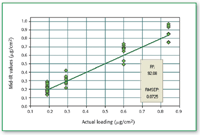
Figure 6. Comparison of predicted amount (using mid-IR) of compound A loaded on metal coupon. The actual amount loaded was determined by HPLC analysis.
It was found that the measurements made on the samples prepared following the smear technique were not consistent. This probably could be due to inhomogenity of samples on the metal coupon. Therefore, only limited measurements were made on the samples prepared using the smear technique. However, consistent spectra were obtained on the samples prepared using spray technique.
The recovery of compound l from metal coupon is nearly quantitative when the amount is greater than 0.4 μg/cm2 (Figures 1â3).
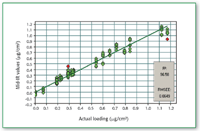
Figure 7. Calibration curve for compound A in the presence of excipients.
Compound A as neat API as well as in the presence of excipients from metal coupon can be determined quantitatively when the concentration is above 0.3 μg/cm2 (Figures 4â8).
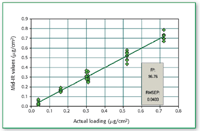
Figure 8. Comparison of predicted amount (using mid-IR) of compound A in the presence of excipients loaded on metal coupon. The actual amount loaded was determined by HPLC analysis.
For compound AE, a calibration curve was generated in the concentration range 0.3â1.12 μg/cm2. An unknown amount of compound AE was loaded on metal coupon and the mid-IR spectra were measured. The predicted values (spectra were collected for each sample on the metal coupon from eight locations and averaged) using the calibration curve were found to be in good agreement with those obtained from HPLC analysis (true values) as shown in Table I.

Table I. Actual versus predicted values for compound AE
Conclusions
A mid-IR fiber optic probe with a grazing-angle head can reliably measure small amounts of organic contaminants on metal surfaces with loadings below 1 μg/cm2. APIs can be analyzed in the presence of excipients on the same surface. There is the potential to build comprehensive calibrations across different surfaces. Thus, a "universal" calibration for a given compound on a range of surfaces might be possible. The technique is rapid, easy to use, and provides a direct, in-situ measurement. The probe requires adjustment, however, to accommodate curved surface geometries of manufacturing equipment. A more reliable sample application technique to generate calibration equations is needed as well.
Acknowledgments
The authors wish to thank Raju Vegesna for helpful discussions and encouragement, Bruker Optics for providing instrumentation, and Dana Kelley at Bruker Optics and Mary Thompson at Remspec for technical help.
References
1. N.K. Mehta, J. Goenaga-Polo, S. Hernandez-Rivera, D. Hernandez, M. Thompson, and P. Melling, Biopharm. 36 (2002).
2. A.A. Urbas and R.A. Lodder, NIR News2, 14 (2003).
3. K. Payne, W. Fawber, J. Faria. J. Buaron, R. DeBono, and A. Mahmood, The Role of Spectroscopy in Process Analytical Technologies (suppl. to Spectroscopy), January 2005.
4. P.R. Griffith and J.A. De Haseth, Fourier Transform Infrared Spectrometry (John Wiley & Sons, Hoboken, NJ, 1986), p. 194.
K. Bal Reddy is a group leader, and Noel Teelucksingh is a senior scientist, at Novartis Pharmaceuticals Corporation, Analytical Research & Development (East Hanover, NJ). E-mail: bal.reddy@novartis.com

Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.