Quantitative Detection of Phenobarbital in an Injectable Solution Based on Surface-Enhanced Raman Spectroscopy
Spectroscopy
This SERS method is rapid, accurate, nondestructive, and easy
A simple method for phenobarbital detection was established. Based on the density functional theory (DFT), the geometry optimization and spectra calculation were performed using Gaussian software on a Becke, 3-parameter, Lee-Yang-Parr (B3LYP) level for phenobarbital. The results from theoretical analysis were compared with experimental results, and the Raman frequencies and their vibrational modes were assigned. A silver colloid surface-enhanced Raman scattering (SERS) substrate, with uniform size and good monodispersity, was prepared, and samples were detected without any pretreatment. The detection conditions were optimized. A linear relationship was obtained between concentration and Raman intensity over the 12–100 µg/mL concentration range. The limit of detection (LOD) was 12 µg/mL, and the recovery rate was between 94.2% and 111.2%. This method shows great practical potential for the identification of phenobarbital.
Phenobarbital is a representative long-acting barbiturate. It has sedative, hypnotic, and anticonvulsant effects. If used improperly (including excessive and long-term use), it may threaten human health. Large doses can inhibit the cardiovascular and respiratory systems, and even can lead to paralysis of the respiratory center of the medulla oblongata. Long-term use can lead to dependence. Psychotropic drugs make the body dependent and can harm human health. Therefore, the use of such drugs should be strictly monitored and controlled.
Raman spectroscopy offers fast, simple, repeatable, qualitative, and quantitative analysis in a noninvasive way. It does not require complex sample preparation, or data analysis (1–3). However, the signal is weak. Thus, surface-enhanced Raman spectroscopy (SERS) is used to overcome problems related to low sensitivity (4,5). It is widely used in pharmaceutical analysis (6,7). Yokoyama and Yamada (8,9) describe a flexible substrate based on SERS for biochemical analysis. However, there is no optimization of the experimental parameters, quantitative analysis, and assignment of the characteristic peaks.
We examined the Raman spectra of phenobarbital using the droplet detection mode (10). Some of Raman characteristic peaks of phenobarbital were assigned, and the Raman peak at 666 cm-1 was used as the basis for stability analysis and quantitative analysis. The experimental conditions were then optimized. The linear quantitative range was 12–100 µg/mL. The main devices and procedures are shown in Figure 1.
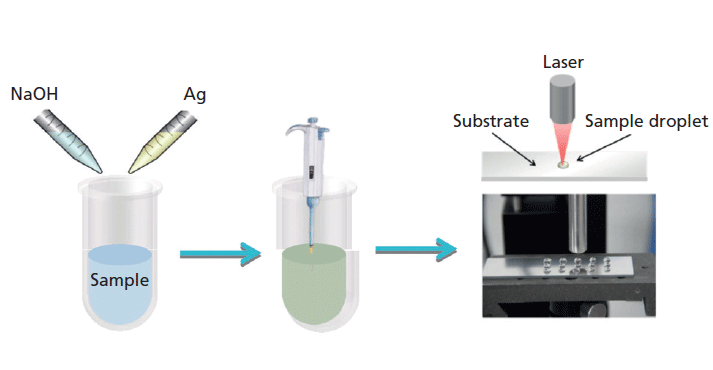
Figure 1: Schematic diagram of the method proposed for the phenobarbital detection process.
Experimental
Reagents
Silver nitrate (AgNO3) and sodium citrate was purchased from Sinopharm Chemical Reagent Co., Ltd. Sodium hydroxide (NaOH) and potassium iodide (KI) were purchased from Xilong Chemical Co., Ltd. An injectable phenobarbital solution (0.1 mg/mL) and phenobarbital powder were purchased from Tianjin Jin Yao Pharmaceutical Co., Ltd. Hydrochloric acid (HCl) was purchased from Tianjin Kermel Chemical Reagent Co., Ltd.
Apparatus
We used a model BWS415-785H portable Raman spectrometer (B&W Tek, Inc.). The laser wavelength was 785 nm. The probe distance was 5.9 mm, and the spectral resolution was less than 3 cm-1. The laser power was 120 mW, and the spectral integration time was 5 s. The data were analyzed by B&W Tek, Inc. embedded software for smoothing and background correction.
Sample Treatment and Preparation of Silver Colloid
The 0.1-mg/mL of phenobarbital injectable solution served as the stock solution. This solution was diluted with deionized water at 12, 20, 30, 50, 70, and 100 µg/mL. The silver nitrate powder was dissolved in deionized water (11) and heated. When the solution boiled, the sodium citrate solution with a mass fraction of 1% was immediately added, and then boiled for 1 h. This solution was then cooled from room temperature to 4 °C, and protected from light.
Results and Discussion
Based on the electromagnetic (EM) enhancement theory, the SERS intensity is dependent on the resonance frequency of the noble metal substrate. The plasmon resonance frequency depends on the size of the nanoparticles, especially for gold and silver nanoparticles (12–15). The morphology and size of the silver nanoparticles were characterized by scanning electron microscopy (SEM), as shown in Figure 2. As can be seen from the map, the silver nanoparticles are spherical and have uniform morphology. The average size of silver nanoparticles was about 50 nm, and its main particle size distribution ranged from 50 to 80 nm by dynamic light scattering analysis.
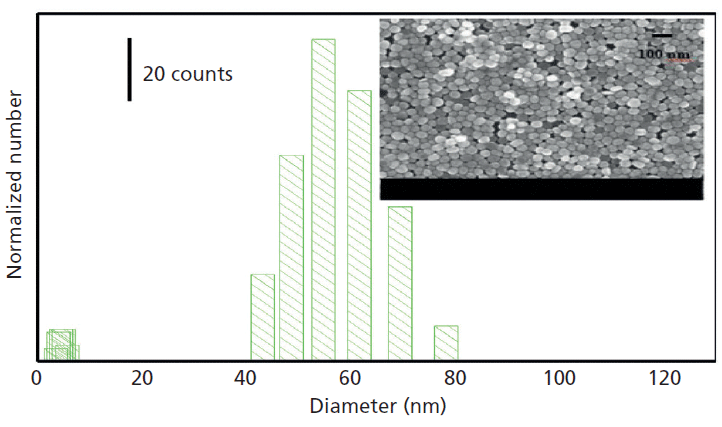
Figure 2: Size distribution of silver nanoparticles (inset: SEM picture of silver nanoparticles).
Raman spectra were performed with the B3LYP hybrid functional with the 6-31g (d, p) basis set. The structure of the optimized phenobarbital molecule is shown in Figure 3. To attribute the Raman characteristic peaks of phenobarbital, we collected the Raman spectra of powder and the SERS spectra of phenobarbital, and compared their Raman spectra with DFT calculation, as shown in Figure 4. It can be seen from the graph that the main Raman peaks in phenobarbital are 625 cm-1, 935 cm-1, 1015cm- 1, 1056 cm-1, and 1189 cm-1. These peaks are basically matched to the Raman peaks of the solid powder and the Raman peaks of the SERS in the aqueous solution. The deviation of displacement is caused by different external conditions. The theoretical model is the case of a single phenobarbital in the vacuum, and the solid and SERS spectra are the results of the solid and liquid phase. The matching and attribution of the specific Raman characteristic peaks of phenobarbital are shown in Table I.
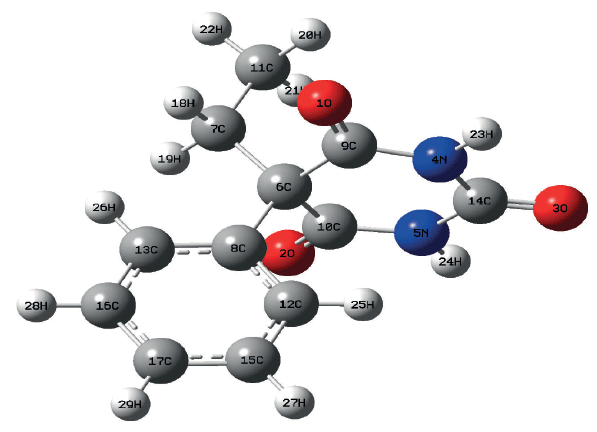
Figure 3: Optimized structure of phenobarbital.
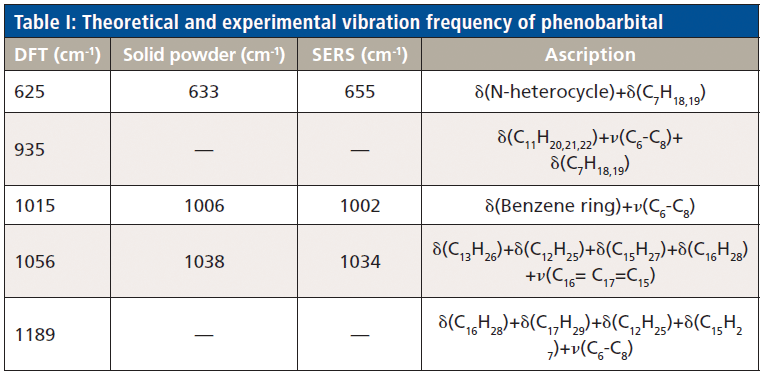
Adding coagulant can change the dispersion state of the nanoparticle sol. This addition breaks the balance potential of the sol system so that the sol particles aggregate (16). It results in more SERS hotspots with good enhancement effects. Thus, we selected 0.1 M (NaOH), acid (HCl) 0.1 M, and neutral (KI) 0.1 M coagulants. The results were collected under the same experimental conditions and show that the Raman spectra of hydrochloric acid coagulant do not induce characteristic peaks (Figure 5). The spectrum with KI coagulation has only subtle peaks at 1002 cm-1 and 1034 cm-1. These phenomena are probably due to the theory of Hofmeister; some ions will destroy the structure of macromolecules and the denaturation strength of chloride ions in the sequence is greater than that of iodine ions, which is consistent with the experimental results. Although a certain degree of migration occurred in the 655 cm-1 position that shifted to 666 cm-1, the spectrum with sodium hydroxide has all strong peaks in phenobarbital. Thus, all subsequent experiments used 0.1 M NaOH as the coagulant. The coagulant, sample, and silver colloid volume ratios were 1:1:1.
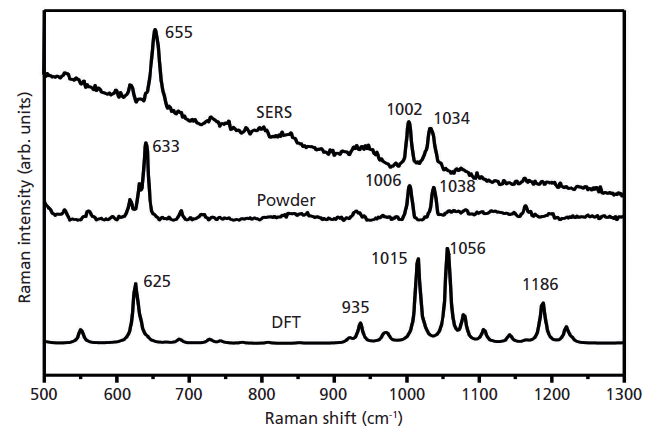
Figure 4: Raman analysis of phenobarbital: theory-calculated, powder, and SERS spectra of an aqueous solution (0.1 mg/mL).
The SERS spectra of different concentrations of phenobarbital were obtained (Figure 6). The laser power was 120 mW, and the integration time was 5 s. All measurements were performed over five replicates and averaged. Above 12 µg/mL, the Raman signal of the sample could still be detected at 666 cm-1. The detection limit of phenobarbital in aqueous solution was 12 µg/mL. In the past, the judgment of the detection limit for the substance is more concentrated on one of the most discernible characteristic peaks. The experiment can also have 666 cm-1 peaks at lower concentration, and 666 cm-1 peaks at lower concentration could be detected. But for the sake of being precise, this article uses 666 cm-1 as the quantitative analysis peak, and the combination of 1002 cm-1 and 1034 cm-1 appears simultaneously as the criterion for determining the LOD.
A stable and repeatable SERS signal is important when using silver aggregates. The 666 cm-1 peak was selected for stability analysis. We selected three different concentrations of phenobarbital solution under the same conditions. These were repeated over 15 experiments in 5 min. The standard deviations at 80 µg/mL and 50 µg/mL were both less than 5% and approximately 5% at 20 µg/mL (Figure 7). Figure 7 shows that the silver aggregates have good repeatability.
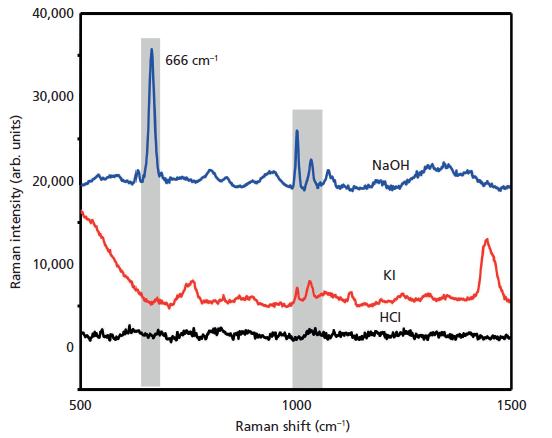
Figure 5: SERS spectra of phenobarbital sodium using different aggregating agents.
Figure 8 shows the linear relationship between the concentration of phenobarbital and Raman intensity at 666 cm-1 from 12 to 100 µg/mL. The fitting equation is y = 1582.6 + 84.896x, where y is the intensity of the Raman peak at 666 cm-1 in the SERS spectrum of the phenobarbital sodium and x is the concentration of phenobarbital sodium. To ensure the accuracy of the data, all spectra were collected five times and averaged. The correlation coefficient is 0.9955, suggesting a good fit.
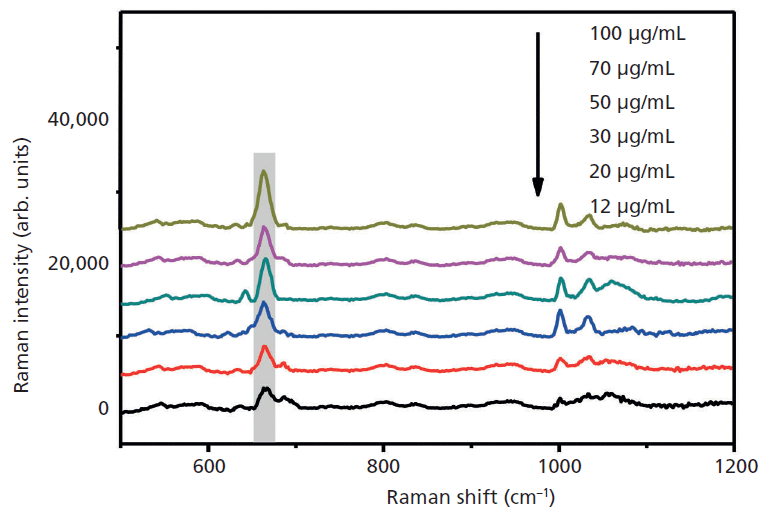
Figure 6: SERS spectra of different concentrations of aqueous phenobarbital.
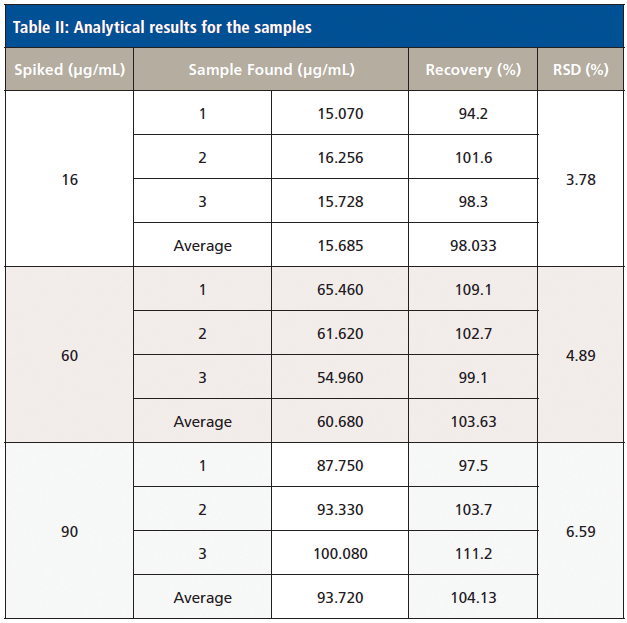
To test the accuracy and reliability of this method, we detected three different concentrations (16 µg/mL, 60 µg/mL, and 90 µg/mL) of the spiked samples. The SERS spectra were collected using a portable Raman spectrometer, and each sample was measured three times. We selected the 666 cm-1 characteristic peak intensity values to fit the formula above. We then calculated the corresponding concentration value, and calculated the corresponding recovery rate and relative standard deviation (Table II). The data indicate that SERS can accurately detect phenobarbital sodium. The recovery rate was 94.2%–111.2%, and the average relative standard deviation is about 5%. SERS detection of phenobarbital is accurate, simple, and efficient. This technology offers real time and rapid detection of phenobarbital.
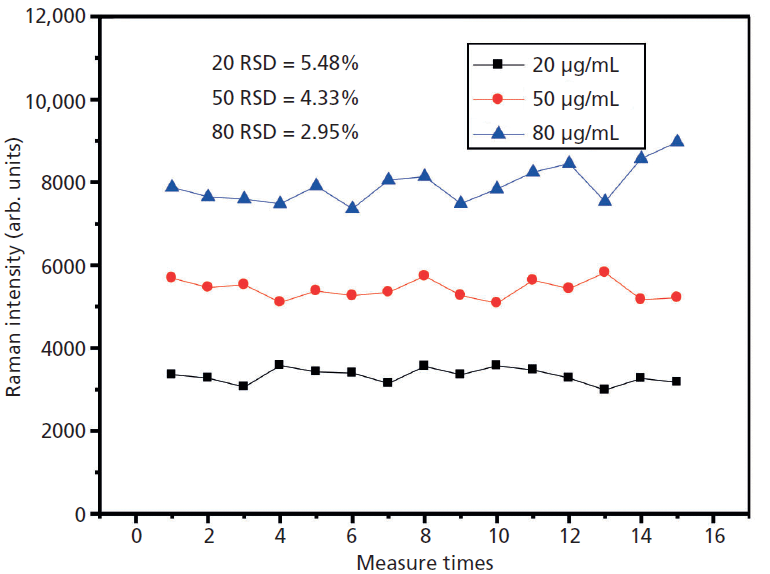
Figure 7: Reproducibility of NaOH-induced silver aggregates in solution.
Conclusions
We used SERS to detect phenobarbital in an injectable solution. First, the hydrogen phenobarbital Raman peaks were assigned to determine the characteristic peak. We then induced aggregation with sodium hydroxide. The aqueous solution of sodium hydroxide increased the SERS signal and lowered the detection limits. We used linear fitting for semiquantitative analysis, and then calculated the recovery rate. The detection limit of phenobarbital is based on the signal at 666 cm-1 in the range of 12–100 µg/mL. The best fit curve is y = 1582.6 + 84.896x (R2 = 0.9955). The recovery rate was 94.2–111.2%. This method is rapid, accurate, nondestructive, and easy. It is useful for the detection of phenobarbital in an injectable solution.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31871873) and the Inner Mongolia Autonomous Region Natural Science Foundation of China (Grant No.2018LH08055).
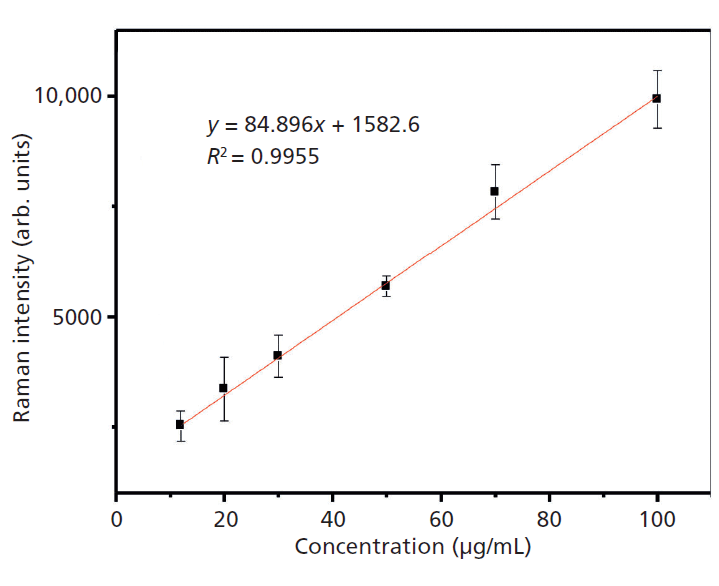
Figure 8: Calibration curve for phenobarbital.
References
(1) K. Hering, D. Cialla, K. Ackermann, T. Dörfer, R. Möller, H. Schneidewind, R. Mattheis, W. Fritzsche, P. Rösch, and J. Popp, Anal. Bioanal. Chem. 390, 113-124 (2008).
(2) C. H. Lee, L. Tian, and S. Singamaneni, ACS Appl. Mater. Interfaces 2, 3429-3435 (2010).
(3) G. Montalvo, L. López-Melero, F. Ortega-Ojeda, M.Á. Peña, and C. Garcia-Ruiz, Anal. Methods 6, 9536-9546 (2014).
(4) A. Campion and P. Kambhampati, Chem. Soc. Rev. 27, 241-250 (1998).
(5) S. Schlucker, Angew. Chem., Int. Ed. 53, 4756-4795 (2014).
(6) L. Bao, X.Y. Sha, H. Zhao, S. Han and W.L.J. Hasi, Anal. Sci, 33, 1237-1240 (2017).
(7) S. Han, L. Bao, M.L. Zhang, X. Lin and W.L.J. Hasi, Anal. Sci, 33, 789-792 (2017).
(8) M. Yokoyama, K. Yamada, T. Nishimura, M. Kido, H. Jeong, and Y. Ohno, Optical Diagnostics and Sensing XV: Toward Point-of-Care Diagnostics, 9332, 933210 (2015).
(9) K. Yamada, T. Endo, H. Imai, M. Kido, H. Jeong, and Y. Ohno, Optical Diagnostics and Sensing XVI: Toward Point-of-Care Diagnostics, 9715, 97150E,(2016).
(10) X. Lin, W.L.J. Hasi, X.T. Lou, S. Lin, F. Yang, B.S. Jia, D.Y. Lin and Z.W. Lu, RSC Advances, 4, 51315-51320 (2014).
(11) P.C. Lee and D. Meisel, J. Chem. Phys. 86, 3391-3395 (1982).
(12) J.L. Hammond, N. Bhalla, S.D. Rafiee, et al. Biosensors 4, 172-188 (2014),
(13) S. Lal, S. Link, N.J. Halas. Nat. Photonics, 1, 641-648 (2007).
(14) G. Frens, Nature, 241, 20-22 (1973).
(15) N.G. Bastus, J. Comenge, V. Puntes, Langmuir, 27, 11098-11105 (2011).
(16) T.Lou, Y.Wang, J.Li, et al. Anal. Bioanal. Chem. 401, 333-338 (2011).
Lin Bao is with the National Key Laboratory of Science and Technology on Tunable Laser at the Harbin Institute of Technology, in Harbin, China, and the College of Physics and Electronics information, at the Inner Mongolia University for Nationalities, in Inner Mongolia Tongliao, China. Yaoye Xu, Hang Zhao, Dianyang Lin, and Wuliji Hasi are with the National Key Laboratory of Science and Technology on Tunable Laser at the Harbin Institute of Technology, in Harbin, China. Siqingaowa Han is with the Affiliated Hospital at the Inner Mongolia University for Nationalities, in Inner Mongolia Tongliao, China. Direct correspondence to: Wuliji Hasi at hasiwuliji19@163.com , Siqingaowa Han at hansiqin@126.com, or Dianyang Lin at dianyanglin@hit.edu.cn

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.