Rapid and Specific Determination of Contaminants, By-Products, and Additives in Insulating Mineral Oils with Tandem Mass Spectrometry
The use of electrospray ionization–mass spectrometry for rapid and specific determination of the widely used metal deactivators Irgamet-30 and Irgamet-39 is demonstrated here.
Insulating oils are often considered the life blood of electrical devices such as transformers. Proper functioning of these devices is essential for uninterrupted electrical power over long periods, often stretching into decades. To ensure longevity of insulating oils and improve certain characteristics additives are often added to the oils. On the other hand, the presence of contaminants can deteriorate the performance of the oils and can cause extensive damage to the electrical devices. To ensure optimal operation of electrical devices, additive and contaminant levels should be periodically monitored. Because of the complex chemical composition of insulating mineral oils, the determination of additives and contaminants is a difficult and often laborious task. However, the task can be made manageable with the use of current state-of-the art analytical instrumentation. This article demonstrates the use of electrospray ionization–mass spectrometry for rapid and specific determination of widely used metal deactivators Irgamet 30 and Irgamet 39. The article also presents specific quantitative determination of a highly corrosive sulfur compound dibenzyl disulfide and its principle nonsulfur by-product bibenzyl with gas chromatography and tandem mass spectrometry (GC–MS-MS).
Corrosion of metals, such as copper, resulting from chemical reactions with sulfur and other chemical species in insulating liquids has been a matter of concern for decades. The presence of corrosive species in general and in particular corrosive sulfur has been linked to failures of electrical equipment used in generation, transmission, and distribution of electrical energy. For this reason, the International Electrotechnical Commission (IEC) standard for mineral insulating oils states that corrosive sulfur compounds shall not be present in unused and used insulating liquids (IEC 60296 clause 6.10) (1).
It is generally accepted that the presence of corrosive sulfur species in mineral oil leads to cuprous sulfide (Cu2S) deposits on the surface of copper conductors. Cuprous sulfide is a semiconductor, and its buildup and subsequent migration into insulating paper over time disrupts the integrity of the insulating paper, which leads to short-circuit faults, sometimes accompanied by windings deformation (2–4). Efforts have been made to assess Cu2S formation resulting from the presence of corrosive sulfur compounds in mineral insulating oils for more than 60 years. Clark and Raab (5) developed a qualitative method for detecting corrosive sulfur in mineral insulating oil during the 1940s. Their approach is still being used with some modifications as standard test methods; for examples, see ASTM D-130 (6), ASTM D-1275 06 (7), DIN 51353 (8), and IEC 62535 (9). However, the standard test methods yield only qualitative results for whether oil contains corrosive sulfur compounds or not under test conditions. Furthermore, the IEC standard test method has been shown to yield false positive results with aged insulating oils and false negative results with oils containing metal deactivators or passivators (10). To overcome the limitations of the current standard test methods for corrosive and potentially corrosive sulfur species, the IEC initiated the development of a quantitative method for a highly corrosive sulfur compound dibenzyl disulfide (DBDS) as well as the development of methods for the quantitative assessment of total corrosive sulfur and specific corrosive species in insulating liquids (11).
A part of this article deals with the specific method for the determination of DBDS and its principle nonsulfur by-product bibenzyl in mineral insulating oils.
This article also deals with the determination of metal deactivators in insulating mineral oils. Metal deactivators or passivators are corrosion-inhibiting chemicals (additives) that interact with metal surfaces and form 0.5–1 nm thick monolayers that limit the access of the corrosive chemical species to the metal surface (12). Common metal passivators added to insulating mineral oils are triazole and benzotriazole derivatives; the two most commonly used are Irgamet 39 and Irgamet 30, which are produced and marketed by BASF (13). Irgamet 39 is a mixture of two isomers, N,N-bis(2-ethylhexyl) 4-methyl-1-benzotriazole-1-methyl amine and N,N-bis(2-ethylhexyl) 5-methyl-1-benzotriazole-1-methyl amine. Irgamet 30 consists of N,N-bis(2-ethylhexyl)-1-triazole-1-methyl amine. Both metal deactivators contain two 2-ethylhexyl chains attached to the triazole or the tolyltriazole moieties. The presence of the 2-ethylhexyl chains in the structure enhances solubility of these compounds in mineral oils. However, the addition also makes the molecules highly unstable and extremely difficult to detect and quantify with methodologies that involve the use of gas chromatography (GC) or liquid chromatography (LC). In fact a well-accepted reversed-phase LC–UV absorption-based method for determination of Irgamet 39 in insulating mineral oil, quantifies only toluyl triazole and not the intact Irgamet 39 (14). However, reversed-phase LC coupled with electrospray ionization (ESI) and tandem mass spectrometry (MS-MS) has been used for quantitative determination of triazole and benzotriazole derivatives in aqueous environmental samples at very low concentrations (15). An LC–ESI-MS-MS method for determination of Irgamet 39 and Irgamet 30 has been reported (16). The method makes use of fragment ions resulting from precursor ion m/z 242 for quantification of both Irgamet 39 and Irgamet 30. The authors assumed that intact Irgamet 30 and Irgamet 39 molecules are separated with reversed-phase LC and the ion m/z 242 results from fragmentation of the parent ion in the ESI source. The ion m/z 242 results from protonation of di 2-ethylhexyl amine, which is present in both metal deactivators. Results obtained in our laboratory indicate that di 2-ethylhexyl amine is released from metal deactivators before their introduction into the LC column. Thus, the approach as described is unlikely to yield results that can be used for specific quantification of Irgamet 30 and Irgamet 39. Therefore, it is apparent that current methods for the detection of these widely used metal deactivators or passivators in mineral insulating oils are unsatisfactory. This article reports a rapid and specific method for the determination of Irgamet 39 and Irgamet 30 in mineral insulating oils with direct infusion ESI-MS, detection limits of the method for both metal deactivators were lower than 1 mg/kg.
Experimental
Materials
Dibenzyl disulfide (DBDS), diphenyl disulfide (DPDS), and bibenzyl (99% purity) were purchased from Sigma Aldrich. White mineral oil was purchased from a local vendor. Insulating mineral oil samples were kindly provided by Sea Marconi. Acetone, acetonitrile, hexanes, iso-octane, and methanol (all Optima grade) were purchased from Thermo Fisher Scientific. Irgamet 30 and Irgamet 39 were kindly provided by BASF. The GC column was purchased from P.J. Cobert Assoc.
Reaction of DBDS with Metallic Copper
Mineral oil was fortified with DBDS at concentrations ranging from 50 to 500 mg/kg. Aliquots of the fortified oil weighing 10 g were transferred to 20-mL borosilicate glass vials, and 1 g of copper granules (~425 µm) was put in the vials along with a 13 × 8 mm PTFE-coated magnetic bar. Vials were purged with argon for 20 min and then were sealed with PTFE-lined crimp caps. The sealed vials were inserted in a thermostated aluminum heating block with 15 cylindrical bores (23 × 56 mm). The size of the bores permitted insertion of the vials in the aluminum block up to their necks. The crimp caps were thus maintained at near-ambient temperature and remained gas tight. The magnetic bars were activated with a magnetic stirrer. The block temperature was maintained at 150 °C. The vials were removed from the heating block after reaction periods varying from 30 min to 8 h.
Quantification of DBDS and Bibenzyl
The concentration of DBDS in mineral oils before and after reaction with copper granules was determined with a gas chromatograph interfaced to a triple-quadrupole mass spectrometer (GC system model 3800; MS system model 320, Varian, [Agilent Technologies]). GC separation was achieved with a 15 m × 0.25 mm fused-silica column with a 95% methyl and 5% phenyl polysiloxane stationary phase. Helium was used as the carrier gas at a constant flow rate of 1.0 mL/min. The injector temperature was maintained at 250 °C, and the transfer line and electron ionization (EI) source were maintained at 250 °C and 200 °C, respectively.
Molecular ions for DBDS (m/z 246), DPDS (internal standard) (m/z 218), and bibenzyl (m/z 182) were mass selected with the first quadrupole and introduced into the second quadrupole with average energy of 30 eV. Collision induced dissociation (CID) in the second quadrupole was assisted with Ar. The product ions for DBDS m/z 91, DPDS m/z 109, and bibenzyl m/z 91 were separated and quantified with the third quadrupole.
Electrospray Ionization–Mass Spectrometry of Irgamet 30 and Irgamet 39
These experiments were carried out with a triple-quadrupole mass spectrometer (model 320, Varian [Agilent Technologies]) equipped with an ESI source. The mass spectrometer was operated in the single-quadrupole mode, whereby ion analysis was performed only with the first quadrupole and the second and the third quadrupoles were operated in the pass all mode. Ions were monitored in the positive ion scan mode with nitrogen as the nebulizing and drying gas. The scan range was 50–450 Da. The ESI source and the analyzer manifolds were maintained at 40 °C. The drying gas temperature was varied from 50 to 200 °C. The needle voltage was varied from 2000 to 5000 V. The drying gas flow rate was maintained at 4 L/min.
To investigate dissociation of Irgamet 30 and Irgamet 39, standard solutions were prepared by dissolving known amounts of commercial formulations as received from BASF in methanol (CH3OH), water (H2O), and deuterium oxide (D2O) followed by serial dilutions with the same solvent. The final concentration of Irgamet 30 and Irgamet 39 in the solutions ranged from 1.0 to 150 mg/L. Next, 5-µL aliquots of the standard solutions were introduced directly into the ESI-MS system through a fixed-volume loop injector. In one set of experiments, four different carrier solvents were used for transporting analyte solutions to the ESI source. The solvents were 100% organic-free deionized water, 100% D2O, 50:50 water–methanol, and 100% methanol. A 15-cm piece (0.127-mm i.d.) of polyether ether ketone (PEEK) tubing was used for connecting the loop injector to the ESI capillary. Carrier solvents were pumped with a 50-mL syringe pump. The volume flow rate of the carrier solvent was varied from 50 to 150 µL/min.
In another set of experiments, trifluroacetic acid was added to the analyte solutions in acetonitrile or methanol. The acid concentration was varied from 0 to 64 ppm by adding varying amounts of trifluoroacetic acid solution (1 mg/mL) to Irgamet 30 or Irgamet 39 solutions. The analyte concentration in acetonitrile or methanol was 70 mg/kg. The carrier solvent consisted of a 50:50 water–methanol mixture, which was pumped with a 50-mL syringe pump. The volume flow rate of the carrier solvent was set at 250 µL/min, the drying gas temperature was set at 150 °C, and the needle voltage was maintained at 4000 V.
After optimization of the ESI-MS parameters, the system was used for quantitative determination of Irgamet 30 and Irgamet 39 in mineral oil. Calibration standards were prepared by fortifying white mineral oil with Irgamet 30 or Irgamet 39 as described earlier. For quantification of the metal deactivators in insulating mineral oils, samples (0.5 g ± 0.01 g) of the oils were taken and mixed with 5 mL of n-pentane, and the solutions were passed through silica gel solid-phase extraction cartridges. Irgamet 30 or Irgamet 39 adsorbed on the silica gel was desorbed with methanol. Then, 5 µL of the extract was introduced into the ESI-MS system. The procedure was used for determining Irgamet 30 in mineral insulating oil samples and a blind Irgamet 30 fortified mineral insulating oil sample obtained from Sea Marconi Technologies. Irgamet 30 or Irgamet 39 in oil was also recovered through liquid–liquid extraction by extracting oil samples diluted in hexanes and extracted with methanol. The oil and hexane layer was decanted, and 200 µL of decafluoropentane (DFP) was added to the methanol extract to facilitate separation of residual hexane and oil from methanol. Aliquots (5 µL) of the methanol layer were introduced into the ESI-MS system.
Results and Discussion
Specific Determination of DBDS and its Byproduct Bibenzyl
Since the discovery of DBDS in commercially available insulating mineral oil, this highly corrosive compound has been found in many unused and used mineral insulating oil samples, particularly in oils from transformers in South America. This sulfur compound has been implicated in a number of transformer failures in that region (17). It has been shown that DBDS is quantitatively converted to cuprous sulfide (see Figure 1) with bibenzyl as the major non-sulfur by-product (18).
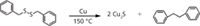
Figure 1: Conversion of DBDS to cuprous sulfide and bibenzyl.
In closed systems, 1 mol of DBDS should yield 2 mol of Cu2S and 1 mol of bibenzyl. However, quantitative assessment of the extent of Cu2S formation is very difficult, and quantitative determination of bibenzyl in mineral oil is also problematic with traditional analytical techniques such as GC–MS or high performance liquid chromatography (HPLC)–UV. Higher throughput and greater specificity were obtained through determination with GC–MS-MS. In this approach, the preliminary separation of components was achieved with capillary GC, and molecular ions for bibenzyl, DBDS, and DPDS (internal standard) m/z 182, m/z 218, and m/z 246 were mass selected with the first quadrupole mass filter and made to undergo CID in the second quadrupole. The CID-produced ions at m/z 91, m/z 109, and m/z 91 were monitored and quantified with the third quadrupole. The specificity and sensitivity achieved with the approach is evident from the extracted ion chromatograms of oil fortified with 4 mg/kg of DBDS (Figure 2).
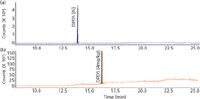
Figure 2: (a) Extracted ion chromatograms (m/z 109) derived from CID of DPDS (internal standard) molecular ion; m/z 218. (b) Extracted ion chromatogram (m/z 91) derived from CID of DBDS molecular ion (m/z 246). DBDS fortification concentration in mineral oil was 4 mg/kg.
The moles of DBDS that had reacted with copper were calculated from the differences in the initial DBDS concentration and DBDS concentration after reaction with copper at 150 °C. Cu2S was quantified as sulfate after its oxidation to cupric sulfate (CuSO4), which was monitored as sulfate (SO42-) with ion chromatography or turbidity measurements. Determination confirmed that 1 mol of DBDS yields 2 mol of SO42- . Results showed that DBDS reacts quantitatively with copper to form Cu2S and the major nonsulfur by-product is bibenzyl. The overall results of these determinations are shown in Figure 3. Because Cu2S formed in an operating transformer cannot be determined, the assessment of DBDS reaction with the copper conductor in closed systems can be made by monitoring bibenzyl in insulating oils.
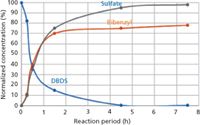
Figure 3: Plots showing the normalized concentrations of DBDS, bibenzyl, and sulfate in mineral insulating oil fortified with DBDS after equilibration with copper granules at 150 °C.
Quantitative Determination of Irgamet 30 and Irgamet 39 by Direct Infusion ESI-MS
Experiments carried out for quantification of Irgamet 30 and Irgamet 39 showed that both the triazole-derived Irgamet 30 and toluyl triazole-derived Irgamet 39 are very unstable in protic solvents. As a result protonated pseudomolecular ions for the two molecules at m/z 323 and m/z 387 were not observed in the ESI spectra. The likely reason for the absence of these ions is rapid dissociation of Irgamet 30 and Irgamet 39 through the retro Mannich reaction (19). Irgamet 30 dissociates into triazole and N,N-bis(2-ethylhexyl) amine when it comes in contact with water or other protic solvents (Figure 4). Similarly, Irgamet 39 dissociates into toluyl triazole and N,N-bis(2-ethylhexyl) amine.
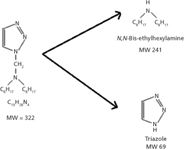
Figure 4: Dissociation of Irgamet 30 in protic solvents.
The dissociation of Irgamet 39 into toluyl triazole and N,N-bis(2-ethylhexyl) amine was supported by its ESI-MS spectrum. The spectrum showed two dominant ions at m/z 134 and m/z 242, resulting from protonation of toluyl triazole and protonation of N,N bis(2-ethylhexyl) amine, respectively. The ratio of ion m/z 135 and ion m/z 134 was in agreement with the expected isotopic ratio for ions containing seven carbon and three nitrogen atoms and the ratio of ion m/z 243 and ion m/z 242 was found to be in agreement with ions containing 16 carbon atoms and one nitrogen atom. Similarly, the ESI-MS spectrum of Irgamet 30 showed two ions; however, the intensity of protonated triazole ion was significantly lower than expected, but ion intensities for ion m/z 70 and ion m/z 242 were sufficiently high to permit quantification of Irgamet 30 in mineral insulating oils at concentrations greater than 10 mg/kg.

Figure 5: ESI-MS spectrum of Irgamet 30 dissolved in D2O and with D2O as the carrier solvent. The spectrum was obtained 30 s after injection.
To gain a better understanding of the dissociation of the two compounds, solutions prepared in D2O were introduced into the ESI-MS system with D2O as the carrier solvent. The Irgamet 30 spectrum obtained under these conditions is shown in Figure 5.
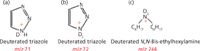
Figure 6: Structures of (a) deuterated protonated triazole ion, (b) deuterated triazole ion, (c) deuterated N,N-bis(2-ethylhexyl) amine ion.
The base ion in the spectrum obtained with the Irgamet 30 solution in 50:50 water–methanol appeared at m/z 242 with a smaller ion at m/z 70, whereas the base ion for Irgamet 30 in D2O solution appeared at m/z 72 (Figure 5). The other significant ions were observed at m/z 71 and 244. The ion at m/z 71 is most likely deuterated protonated triazole (Figure 6a) and the ion at m/z 72 is doubly deuterated triazole ion (Figure 6b). The ion at m/z 244 is doubly deuterated N,N-bis(2-ethylhexyl) amine ion (Figure 6c).
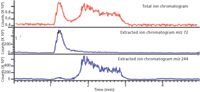
Figure 7: TIC and extracted ion chromatograms for Irgamet 30 dissolved in D2O.
The reason for change in the Irgamet 30 ESI-MS spectrum became apparent after examining the pseudo extracted ion chromatograms obtained by direct infusion of solution in D2O with D2O as the carrier solvent (Figure 7). The term "pseudo extracted ion chromatograms" is used to emphasize the fact that no chromatographic column was used during the analysis. The extracted ion chromatograms obtained under these conditions showed that ions m/z 72 and m/z 244 for triazole and N,N-bis(2-ethylhexyl) amine, respectively, emerged at different times from the PEEK tubing despite the fact that no chromatography column was present in the system. The difference in emergence time can be attributed to the differences in the solubility of triazole and N,N-bis(2-ethylhexyl) amine in D2O and their different affinity for the PEEK capillary surface, which acted as a chromatographic column. The separation clearly shows that Irgamet 30 dissociated in the solution before its introduction into the ESI source. The more polar and smaller triazole molecule migrated faster in the polar carrier than the molecule with long hydrocarbon chains. This observation was supported by the ESI-MS spectrum obtained around the apex of the m/z 244 peak (Figure 8). The doubly deuterated triazole ion m/z 72 was not observed in the spectrum because Irgamet 30 dissociated and triazole did not coelute with the N,N-bis(2-ethylhexyl) amine from the PEEK capillary.

Figure 8: ESI-MS spectrum of Irgamet 30 dissolved in D2O and with D2O as the carrier solvent. The spectrum was obtained 70 s after injection.
Similar results were obtained with Irgamet 39 dissolved in D2O. Toluyl triazole was readily separated from the N,N-bis(2-ethylhexyl) amine. The spectrum obtained showed the presence of base ion at m/z 136, a weaker ion at m/z 135, the isotopic ion at m/z 137, and an ion at m/z 244 (Figure 9).

Figure 9: ESI-MS spectrum of Irgamet 39 dissolved in D2O and with D2O as the carrier solvent. The spectrum was obtained 30 s after injection.
The ion at m/z 135 represents deuterated toluyl triazole, the base ion at m/z 136 represents the doubly deuterated toluyl triazole ion, and the ion at m/z 244 represents doubly deuterated N,N-bis(2-ethylhexyl) amine. As expected, the later spectrum contained only the singly deuterated and the doubly deuterated N,N-bis(2-ethylhexyl) amine ions m/z 243 and m/z 244 (Figure 10).

Figure 10: ESI-MS spectrum of Irgamet 39 dissolved in D2O and with D2O as the carrier solvent. The spectrum was obtained 60 s after injection.
Results clearly show that both Irgamet 30 and Irgamet 39 dissociate readily in protic solvents. However, these additives can be determined in insulating oils by direct infusion of extracts into the ESI-MS system. Detection limits down to 1 mg/kg can be readily obtained with a linear dynamic range of more than two orders of magnitude. The precision of the approach was evaluated with six replicate analyses at three concentrations: 10, 30, and 100 mg/kg. The average relative percent standard deviation was 4.8%. A blind fortified check mineral insulating oil sample was obtained from Sea Marconi Technologies. The average Irgamet 30 concentration in six replicate samples was 33.8 mg/kg, and the fortification level reported by Sea Marconi Technologies was 30 mg/kg. The result clearly indicates that the approach can be used for rapid determination of Irgamet 30 and Irgamet 39.
Conclusions
The results of experiments carried out during the study clearly demonstrate that sensitive and specific quantification of both the corrosive sulfur compound DBDS and its principal nonsulfur by-product bibenzyl in insulating mineral oils can be achieved with GC–MS-MS with minimal sample preparation. Under suitable conditions bibenzyl can be used as diagnostic tool for assessing DBDS-related Cu2S formation and potential risk of transformer malfunction.
Results also show that commonly used triazole and toluyl triazole derived metal deactivator Irgamet 30 and Irgamet 39 can be readily determined in mineral insulating oils with ESI-MS through direct infusion of the oils extracts. Quantification is rapid, sensitive, and specific over a wide range of concentrations.
Acknowledgments
The study was supported in part by the Center for Environmental Science and Technology — Missouri S & T (a campus of the University of Missouri) and Sea Marconi Technology, Turin, Italy.
References
(1) IEC 60296: Fluids for Electrotechnical Applications — Unused Mineral Insulating Oils for Transformers and Switchgear.
(2) A.C.M. Wilson, Insulating Liquids: Their Use, Manufacture and Properties (IEEE, New York and UK, 1980).
(3) R.L. Lewand, Corrosive Sulfur in Transformer Systems, Chemist's Perspective (Doble Engineering Company, Neta World, USA, 2003).
(4) J.R. Smith, Industry Applications Society Annual Meeting (IAS) – IEEE, Proceedings of the Industrial Applications, 1-4 (2010).
(5) F.M. Clark and R.L. Raab, "The Detection of Sulfur Compounds in Mineral Transformer Oil," ASTM Symposium Publication, 1201–1210 (1948).
(6) American Society for Testing and Materials, D130 Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test.
(7) American Society for Testing and Materials, D 1275 06 Standard Test Method for Corrosive to Sulfur in Electrical Insulating Oils.
(8) Standard Test Method DIN 51353: Testing of Insulating Oils; Detection of Corrosive Sulfur; Silver Strip Test.
(9) IEC 62535: Test Method for Detection of Potentially Corrosive Sulfur in Used and Unused Insulating oil (October 2008).
(10) V. Tumiatti, M. Tumiatti, R. Maina, and C. Roggero, Proceedings of the GCC-Cigre, Exhibition and Conference, Doha, Qatar, 2009.
(11) IEC 62697-1: Standard Test Method for Quantitative Determination of Dibenzyl Disulfide in Insulating Liquids (August 2012).
(12) M. Levin, P. Wiklund, and H. Arwin, Appl. Surf. Sci. 254, 1528–1533 (2007).
(13) http://www.performancechemicals.basf.com.
(14) Detection of CIBA Irgamet 39 in insulating mineral oil, Cigre WG A2.32TF 02. Report of the Working Group A2. Task Force 32 (2002)
(15) S. Weiss and T. Reemtsma, Anal. Chem. 77, 7415–7420 (2005).
(16) A. Schaut, S. Autru, A. De Rop, and S. Eeckhoudt, IEEE Trans. Dielectr. Electr. Insul. 19 (1) (2012).
(17) N. Dominelli, S. Kovacevic, E. Hall, and M. Lau, "Insulating Materials Session," Proceedings of the 74th Annual Conference of Doble Clients, Boston, Massachusetts, 2007.
(18) S. Kapila, V. Tumiatti, M. Tumiatti, C. Roggero, R. Seemamahannop, and R. Andersson, Proceedings of the My Transfo Meeting, Turin, Italy, 2008.
(19) C. Mannich, W. Krösche, Archiv der Pharmazie 250, 647–667 (1912).
Carlo Roggero, Jinyu Du, R. Seemamahannop, and S. Kapila are with the Center for Environmental Science and Technology, Missouri S&T (University of Missouri), in Rolla, Missouri. V. Tumiatti and Michela Tumiatti are with the Sea Marconi Technologies, in Turin, Italy. Direct correspondence to: kapilas@mst.edu

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.