A Simple and Effective ICP-MS Method Capable of Detecting Trace and Major Elements in Milk Samples
Special Issues
The method described here is a simple method for detection of a wide spectrum of metals and for quantitative analysis over a wide dynamic range of elements, including both toxic metals and those metals essential for health and well-being.
To address the challenges associated with a broad, complicated food and beverage supply chain, innovative solutions for contaminant detection are continually being sought. Easy-to-operate instrumentation and methodology can be adopted over the entire food supply chain, allowing contaminated food samples to be identified more readily. Here, we describe a simple method capable of identifying and quantifying a diverse range of elements, including trace metals that are toxic in low concentrations, and also the major elements that are essential to healthy sustainable life. Inductively coupled plasma mass spectrometry (ICP-MS) is the definitive technique used to identify and quantify potential metal contaminants; here it has been applied to the metals present in samples of milk powder and evaporated or condensed milk. The ICP-MS method used enabled the detection of metals with quantitation limits to the order of parts per billion. It was found that all milk samples contained elemental concentrations below those levels directed by regulation, with milk powders containing the highest concentrations of major metal elements.
Mass spectrometry has increasingly become associated with the detection of contaminants and nutrients in food, overtaking other less common methods of detection. Under the umbrella of mass spectrometry are inductively coupled plasma mass spectrometry (ICP-MS) and liquid chromatography and tandem mass spectrometry (LC–MS/MS), which have both been adopted increasingly by researchers throughout the world to identify and quantify contaminants and nutrients in food. As demonstrated by the 2017 fipronil contamination scare (1), there is an industrial necessity to provide reliable analysis methods that can be applied by any food scientist within any processing facility. In tandem with the detection of contaminants, food must also be nutritious, supplying consumers with the essential components of life, such as the metals that are involved in important biological processes.

(PHOTO CREDIT: JOE ZUGIC PHOTOGRAPHY)
Elements are the building blocks of life, and we need to ensure that we receive the right mixture of elements at the appropriate dosage for our continued sustainable development and healthy growth. Of the 118 elements on the periodic table, the majority are metals. For humans, receiving the appropriate levels of certain metals is vital to our existence. Iron, for instance, is an essential component of hemoglobin, and assists in transporting oxygen throughout the body. Other major elements include sodium, potassium, magnesium, copper, and zinc, all of which facilitate a range of biological functions and roles within the human body, making them nutritionally essential. Therefore, in order to function appropriately, we need to be sure we are accumulating the right mixture of elements at the appropriate levels. Dosage of any element is important; an element must be present at a safe enough level to provide functionality, but not so high that it becomes toxic. This means reliable quantitation is essential.
However, there are also those elements that are toxic even in low concentrations. For instance, the metalloid arsenic (2), and heavy metals such as mercury (3), lead (4), and cadmium (5) have been associated with a variety of health issues. While heavy metals occur naturally, their release into the environment is often a result of anthropogenic activities that are commonly industrially related (6), which can facilitate their dispersion into the food chain. To address the variety of health issues associated with heavy metal contamination, government or independent health experts have imposed maximum levels of metals in foodstuffs to further protect the public.
ICP-MS itself is becoming renowned as the method of choice for metal analysis across a variety of applications from pharmaceutical development and cancer research (7) to environmental and food analysis (8,9). Specifically considering its use in the food industry, ICP-MS has been used across a variety of applications such as arsenic detection in rice (10). One of the specialties of ICP-MS is its intrinsic use of plasma that completely ionizes the entire sample, ensuring the detection of all metals or analytes. Methods such as atomic absorption spectroscopy and ICP-OES have been successfully applied to the analysis of metal elements within samples of food (11). However, both these methods lack either the multi-element detection capabilities or the sensitivity that ICP-MS offers.
For food samples, the ability to identify and quantify the various metal elements within a given sample is essential both for contaminant detection and accurate nutrient reporting. Here, we describe a fast and simple method capable of detecting a dynamic range of elements to a high level of reliability and accuracy within various samples of milk with variable fat levels. Milk is well known as a source of nutrition in the early development of children. During these early stages, confidently knowing the quantities of nutritional elements being ingested is critical to healthy growth. This underpins the need for a useful methodology that can provide simple solutions to food scientists across the globe (12).
Experimental
Six samples of milk were purchased from local stores for analysis. Samples were chosen to provide a concise coverage of various types of milk with a varied concentration of fat. The forms of milk used were: skimmed milk powder (non-fat), light evaporated milk (4% fat), evaporated milk (9% fat), sweetened condensed milk, skimmed milk (ultra-high temperature (UHT) milk, <0.5% fat), and semi-skimmed milk (UHT, < 2% fat). For experimental controls, three certified reference materials were also obtained. Two European Reference Materials (ERMs) were obtained from the Joint Research Centre of the European Commission, denoted as ERM-BD 150 and ERM-BD 151. The National Measurement Institute of Japan (NMIJ) sample, NMIJ 7512-a, was obtained from GL Sciences B.V.
Milk samples of varying weights (5 g of UHT milk, 2 g evaporated milk, 1 g condensed milk, and 0.5 g milk powder) were digested with a combination of concentrated 2.5 mL nitric acid (Fluka, TraceSelect Ultra) and 2.5 mL 30% hydrogen peroxide (Sigma-Aldrich, H2O2 ≥30%, for Ultratrace analysis). Samples were made up to equivalent volume by the addition of water, which was altered to account for the varying concentrations in the different types of milk (0 mL for UHT milk, 3 mL for evaporated milk, 4 mL for condensed milk, and 5 mL for milk powder). Samples were then heated using a Titan MPS Microwave Sample Preparation System using standard 75 mL vessels. Digests were transferred to 50 mL autosample tubes and spiked with 10 µL of 1000 mg/L gold solution before being made up to 50 mL with deionized water.
Analyses were performed using the NexIon 2000 P ICP-MS instrument (PerkinElmer) with sample detection achieved using a collision mode to reduce elemental interference. The technical parameters for the instrument are reported in Table I. No modifications were made to the instrument and the default sample introduction system was used.
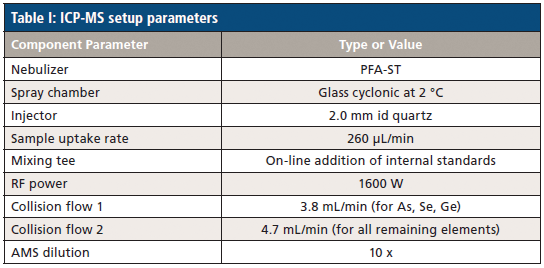
Results and Discussion
Detection of Major and Trace Elements in Milk Samples
Before we discuss the analysis of store-bought milk samples in detail, the methodology of sample preparation and comparisons between the control and current methods should be explained in further detail. The first important step in preparing samples for analysis is the removal of the fat content. Milk samples vary in the quantity of fats. For instance, skimmed milk powder is considered to contain no fat, whereas evaporated milk typically contains 9% fat. Excess fat in the samples will negatively affect the performance of the ICP-MS. Therefore, excess fat must be broken down prior to sample analyses to ensure quality and consistency between samples and the uninhibited detection of the elements.
To break down fats, the sample is digested. While there are several conceivable methods of achieving this, the method employed here involves a mixture of nitric acid and hydrogen peroxide. This mixture breaks down the proteins and makes the sample more viscous. It is then necessary to heat samples, which facilitates digestion. The method used here involves the use of a microwave to ensure uniform heating across the entire sample. An alternative could involve using heating blocks, however, this might result in uneven heating.
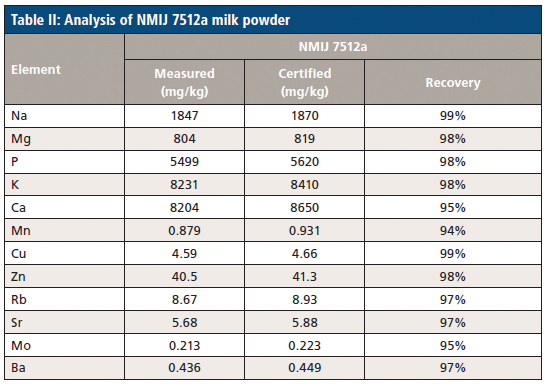
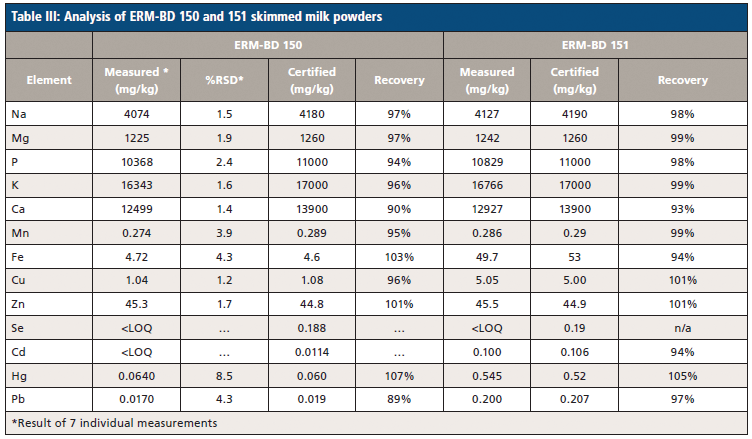
Following digestion, the certified reference materials are analyzed to demonstrate the accuracy of the methodology being used. The results of NMIJ 7512-a milk powder sample and ERM-BD 150 and ERM-BD 151 skimmed milk powders are reported in Tables II and III, respectively. Recovery rates (the difference between the measured and certified samples) are in the range of 89–107%, 93–105%, and 94–99% for the three CRM samples, respectively. These excellent recovery rates demonstrate an overall agreement between the control experimental levels and the presently employed methodology, meaning we can be confident in the elemental breakdown of the milk samples.
Next, the limits of quantitation (LOQ) must be discussed further. These limits are essential in order to identify the limitations of the instrumentation and determine whether results obtained are trustworthy. The limits of UHT milk samples were calculated following recommendations of the Commission Regulation (EC) No 333/2007 (13) as 10 times the standard deviation of 10 consecutive blank measurements. Milk powder LOQs were taken as 100 times the limit of the UHT samples.
One of the challenges of using ICP-MS is in the potential for the interference of elements that can lead to false positive element detection. This is a common issue, and is the result of cross-reactivity between various other species. For instance, 40Ar16O is a common interfering species of 56Fe (14). In this example, to prevent false detection of iron, the argon oxide analyte needs to be removed. This is where collision cells become useful. Collision cells work on the principle that the interfering ion, in this case ArO+, is much larger than the analyte, in this case Fe+. Upon ionization, both species travel through a stream of inert gas. The larger analytes are more likely to collide with gas particles that remove them from the mass spectrometer instrument. The intensity of all analytes is reduced, including the desired analyte as a result of a low proportion of collisions with the inert gas stream. However, any analyte intensity reduction is offset by the reduction in the intensity of the interference.
Analytes of any form are then compared against regulation approved levels to ensure that the food is safe. Levels of specific metals must be within defined limits, typically sub parts per million. As defined by the Commission Regulation (EC) 1881/2006, the regulated levels for lead and tin in milk are 0.02 and 50 mg/kg, respectively (15). In this instance, it was possible to detect metals to the level of singular parts per billion, well below the relevant limits. The measured LOQs for lead and tin in milk powders are 0.0017 and 0.012 mg/kg, respectively, several orders of magnitude below the limits. In addition, the LOQ for mercury was found to be 0.0083 mg/kg, indicating the potential to detect ultralow levels of mercury in milk. LOQs to this order of magnitude below the regulatory limits add credence and confidence to the results of the experiment.
Using the methodology outlined in the experimental section, the store-bought samples were analyzed to identify and quantity a range of elements. This included the so-called essential major elements such as sodium, magnesium and calcium, amongst others, and also the toxic trace elements such as mercury, cadmium and lead. Measured concentrations for the various samples of milk are reported in Figure 1. Any sample with a reported measurement below the LOQ has not been plotted.
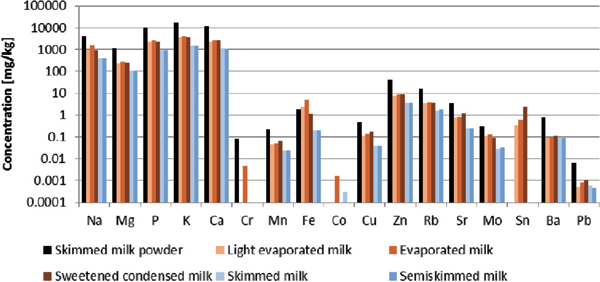
Figure 1: Results of analysis for store-bought samples (milk powder in black; evaporated milks in shades of orange; UHT milks in shades of blue-grey). Values below the LOQ have not been plotted.
From Figure 1, it is clear that the concentrations of major elements are highest in milk powder samples, followed by condensed and evaporated milk samples and finally are lowest in the UHT samples. Certain spikes in samples can potentially be explained as a result of the storage method of the milk. For instance, the elevated levels of tin in evaporated and condensed milk have been hypothesized to be a result of their storage in tin-steel cans. This phenomenon is not unique to milk, and in fact has previously been detected in other examples of canned foods (16).
Fundamentally, this method offers an advantageous analytical technique for the analysis of metal elements over previous instrumentation and methodology due to its twofold advantages. First, it presents a simple method to reliably quantitate a dynamic range of elements that include both trace toxic metals and those essential for health and well-being within milk samples. Secondly, it demonstrates the ability to implement a relatively simple methodology for the employment of metals detection.
Summary
This method provides an analytical technique for the analysis of metal elements in milk demonstrating a twofold advantage. First, it provides a simple method for quantitative analysis over a wide dynamic range of elements, including both toxic metals and those metals essential for health and well-being. Secondly, it demonstrates a simple methodology for a wide spectrum detection of metals.
References
(1) European Commission Fipronil Factsheet, 2017, available at: https://ec.europa.eu/jrc/en/publication/brochures-leaflets/fipronil-eggs-factsheet-december-2017.
(2) The EFSA Journal, 2009, 7, https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.1351.
(3) The EFSA Journal, 2004, 34, 1 https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2004.34.
(4) The EFSA Journal, 2010, 8, 1570 https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2010.1570.
(5) The EFSA Journal, 2009, 980, 1
.
(6) J. Nriagu, Science272, 223-224 (1996).
(7) E.M. Brouwers, M. Tibben, H. Rosing, J.H.M. Schellens and J.H. Beijnen, Mass Spectrometry Reviews27, 67 (2008).
(8) D. Pröfrock, Appl. Spectrosc.66, 843 (2012). http://aaes.auburn.edu/wrc/wp-content/uploads/sites/108/2015/11/ICP-MS-review-.pdf.
(9) The Analysis of Food Substances by ICP-MS, 2008 http://www.spectroscopyonline.com/analysis-food-substances-icp-ms.
(10) PerkinElmer application note, Arsenic Speciation Analysis in White Rice by HPLC/ICP-MS Using the NexION 300D/350D, 2014, available through the following link: http://www.perkinelmer.co.uk/lab-solutions/resources/docs/NexION300D-ArsenicSpeciationInWhiteRice.pdf.
(11) PerkinElmer application note, Determination of Lead and Cadmium in Foods by Graphite Furnace Atomic Absorption Spectroscopy, 2014, available through the following link: http://www.perkinelmer.co.uk/lab-solutions/resources/docs/APP_PinAAcle-900H-Lead-Cadmium-in-Food-011965_01.pdf.
(12) R. Pilarczyk, J. Wójcik, P. Czerniak, P. Sablik, B. Pilarczyk and A. Tomza-Marciniak, Environ. Monit. Assess.185, 8383 (2013).
(13) Commission Regulation (EC) No 333/2006, Laying down the Methods of Sampling and Analysis for the Official Control of the levels of Lead, Cadmium, Mercury, Inorganic Tin, 3-MCPD and benzo(a)pyrene in Foodstuffs, Official Journal of the European Union, European Commission, 2007, L 88, 29 https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1529508094989&uri=CELEX:32007R0333.
(14) PerkinElmer technical note, The 30 minute guide to ICP-MS, available through the following link: https://www.perkinelmer.com/lab-solutions/resources/docs/TCH-30-Minute-Guide-to-ICP-MS-006355G_01.pdf.
(15) Commission Regulation (EC) No 1881/2006, Setting Maximum Levels for Certain Contaminants in Foodstuffs, Official Journal of the European Union, European Commission, 2006, L 364, 5 https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1529507427510&uri=CELEX:02006R1881-20170728.
(16) J.C. Sherlock and G.A. Smart Food Additives and Contaminants3, 277-282 (1984).
Helmut Ernstberger is an inorganic field application specialist at PerkinElmer, in Milan, Italy. Fadi Abou-Shakra is the ICP-MS portfolio director at PerkinElmer in Toronto, Ontario, Canada. Direct correspondence to: Vaughan.Langford@syft.com

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.