3D ZnII-Based Coordination Polymer Shows High Sensitivity in Fluorescent Detection of Nitroaromatic Compounds
A newly developed 3D ZnII-based coordination polymer demonstrates exceptional sensitivity in fluorescently detecting nitroaromatic compounds. This research offers potential applications in the field of chemical sensing and provides insights into the interactions between coordination polymers and target molecules.
Water-stable coordination polymers (CPs) are attracting significant attention because of their potential applications in various fields. In a recent study published in Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, researchers at Xinyang Normal University in China have successfully synthesized a 3D ZnII-based coordination polymer with remarkable fluorescent sensing properties for nitroaromatic compounds (1).
3D image of triaminotrinitrobenzene skeletal formula - molecular chemical structure of aromatic explosive TATB isolated on white background | Image Credit: © kseniyaomega - stock.adobe.com
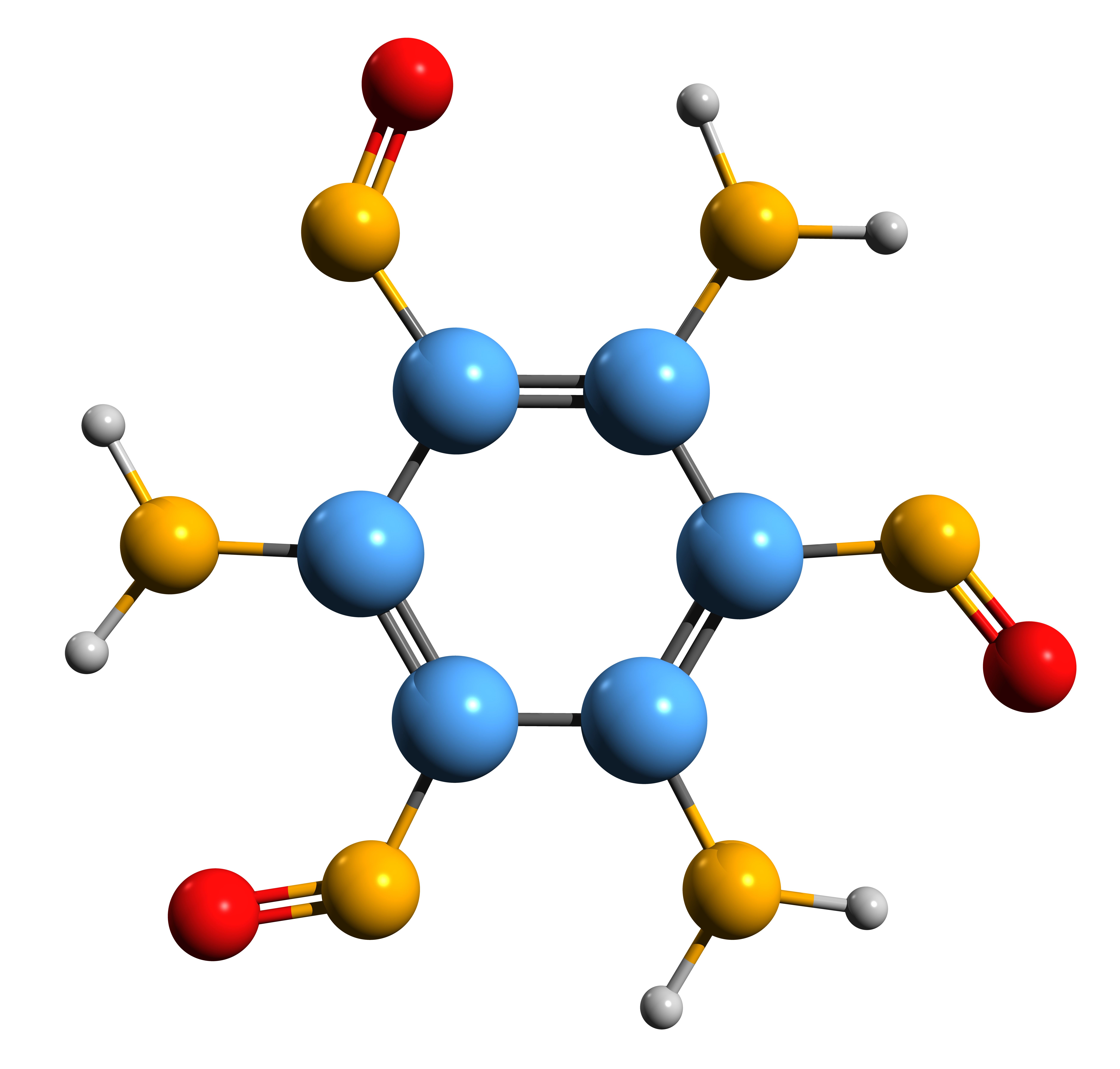
The synthesized compound, named [Zn2L(atez)(H2O)2] (compound 1), exhibits excellent photophysical behavior and water stability. It was prepared through a solvothermal reaction of Zn ions with a multicarboxylate ligand (H3L = 4-(2',3'-dicarboxylphenoxy)) and a nitrogen-containing linker (atez = 5-aminotetrazole). The structure of compound 1 reveals a hierarchically pillared 3D network based on 2D multicarboxylate-ZnII layers connected by atez ligands.
The researchers investigated the sensing capabilities of compound 1 and found that it can selectively and sensitively detect nitroaromatic compounds in water suspension by exhibiting a fluorescence quenching effect. Notably, it displayed high specificity in detecting nitrobenzene (NB) and 2,4,6-trinitrophenol (TNP) with remarkable quenching constants (KSV = 7.5 × 104/M for NB and KSV = 1.9 × 105/M for TNP) and low limits of detection (LOD = 0.93 μM for NB and LOD = 0.36 μM for TNP).
The researchers identified that the sensing mechanism behind these processes involves both fluorescence resonance energy transfer (FRET) and photoinduced electron transfer (PET) between the coordination polymer and nitroaromatic molecules. This finding not only contributes to the understanding of the interaction between CPs and nitroaromatic compounds but also offers a potential novel fluorescence probe for their detection.
FRET and PET are two mechanisms that play crucial roles in energy transfer processes within molecular systems. FRET occurs when energy from an excited fluorophore (donor) is transferred non-radiatively to a nearby acceptor molecule, resulting in the emission of fluorescence from the acceptor. This transfer of energy is distance-dependent and relies on the spectral overlap between the donor emission and acceptor absorption spectra. On the other hand, PET involves the transfer of an electron from the excited state of a donor molecule to an acceptor molecule, leading to quenching of the donor fluorescence. PET can occur when the energy levels of the excited donor and acceptor match, allowing for efficient electron transfer.
The successful synthesis and characterization of the 3D ZnII-based coordination polymer, along with its promising fluorescent sensing properties for nitroaromatic compounds, demonstrate the potential of CPs as effective tools for molecular detection. This research opens up new avenues for improving the applications of fluorescent CPs and paves the way for further advancements in the field of chemical sensing and environmental monitoring.
Reference
(1) Wang, Y.-N.; Xu, H.; Wang, S.-D.; Feng, W-Y.; Mo, Y.; Bai, J.-T. Qiu, Q.-C.; Wang, Y.-T.; Zhang, M.-H.; Yang, Q.-F. 3D ZnII-Based coordination polymer: Synthesis, structure and fluorescent sensing property for nitroaromatic compounds. Spectrochimica Acta Part A: Mol. Biomol. Spectrosc. 2023, 297, 122708. DOI:10.1016/j.saa.2023.122708
New Fluorescence Model Enhances Aflatoxin Detection in Vegetable Oils
March 12th 2025A research team from Nanjing University of Finance and Economics has developed a new analytical model using fluorescence spectroscopy and neural networks to improve the detection of aflatoxin B1 (AFB1) in vegetable oils. The model effectively restores AFB1’s intrinsic fluorescence by accounting for absorption and scattering interferences from oil matrices, enhancing the accuracy and efficiency for food safety testing.
Tracking Molecular Transport in Chromatographic Particles with Single-Molecule Fluorescence Imaging
May 18th 2012An interview with Justin Cooper, winner of a 2011 FACSS Innovation Award. Part of a new podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2012 ? the Great Scientific Exchange, the North American conference (39th Annual) of FACSS.
New Fluorescent Raman Technique Enhances Detection of Microplastics in Seawater
November 19th 2024A novel method using fluorescence labeling and differential Raman spectroscopy claims to offer a more efficient, accurate approach to detect microplastics in seawater. Developed by researchers at the Ocean University of China, this method improves both the speed and precision of microplastic identification, addressing a key environmental issue affecting marine ecosystems.