Analysis of a Bispecific Antibody Using SEC–MS
Special Issues
This article describes a workflow using nontargeted liquid chromatography–tandem mass spectrometry (LC–MS/MS) for reliable compound identification.
More potent formats of monoclonal antibodies (mAbs), such as bispecific antibodies (bsAbs), are on the rise in the area of biotherapeutics. Characterization of bsAbs is essential to ensuring product safety and efficacy. Size-exclusion chromatography (SEC) coupled with mass spectrometry (MS) is increasingly being used to identify the accurate molecular mass of biomolecules, including bsAbs. SEC–MS, however, requires the use of mobile phases that do not contain high concentrations of nonvolatile salts and the use of columns that do not exhibit particle shedding, both of which will interfere with the MS signal response.
In this application note, a bispecific T cell engager (BiTE®) consisting of two single-chain variable fragments (scFvs) recombinantly linked by a nonimmunogenic five-amino-acid chain was analyzed by SEC–MS using a TSKgel® UP-SW3000, 2 μm column.
Experimental HPLC Conditions
Column: TSKgel UP-SW3000, 2 μm, 4.6 mm ID x 30 cm
HPLC Instrument: Nexera® XR UHPLC system
MS Instrument: Q Exactive™ Plus
Mobile phase: 20 mmol/L ammonium acetate, 10 mmol/L ammonium bicarbonate; pH 7.2
Flow rate: 0.35 mL/min
Detection: UV @ 280 nm
Temperature: 30 °C
Injection vol.: 5.0 μL
Samples: BiTE, 0.3 mg/mL (Creative Biolabs)
Ionization mode: Electrospray ionization, positive mode
MS mode: Scanning, m/z 800–6000
Results and Discussion
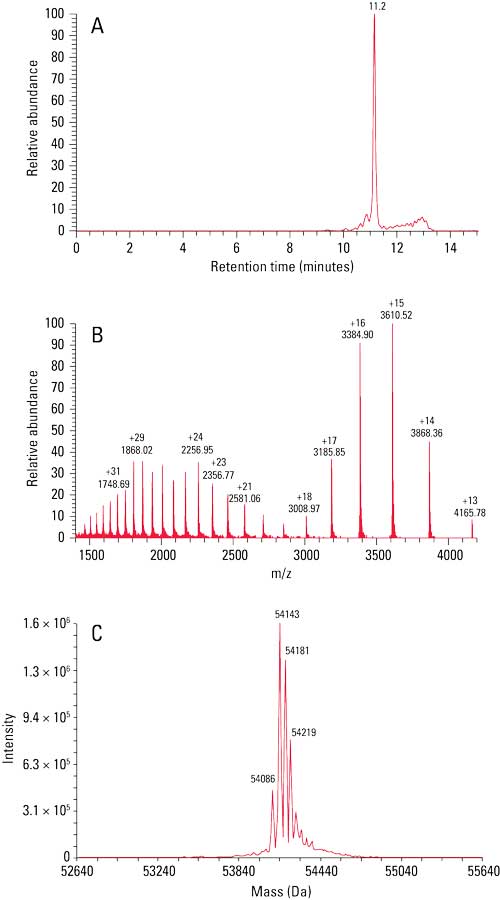
The ~55 kDa BiTE and parent mAbs (data not shown) were subsequently injected onto a TSKgel UP-SW3000 column coupled to a mass spectrometer for molar mass determination. Figure 1 shows the (a) total ion chromatogram, (b) mass spectrum, and (c) deconvoluted mass spectrum of the BiTE. A main peak can be seen at m/z 54,143; adjacent peaks at m/z 54,181, 54,219, and 54,086 correspond to different salt adducts.
Figure 1: Figure 1: SEC–MS analysis of the BiTE. Accurate molar mass of the BiTE was identified as 54.1 kDa via SEC–MS.
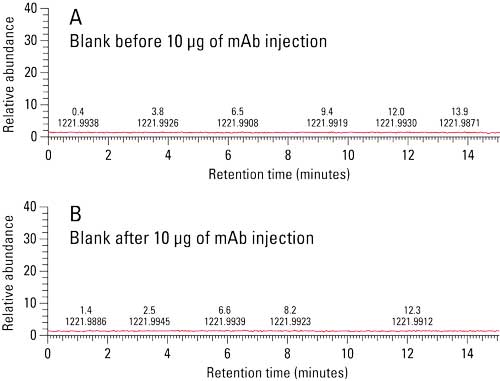
Prior to analysis, a blank injection was run in order to assess column particle shedding. Figure 2a shows the total ion chromatogram of a blank injection. MS data indicates that there is no shedding from the TSKgel UP-SW3000 column prior to sample injection. Additionally, a blank injection was run between each of the sample injections in order to monitor sample carryover. Figure 2b shows the total ion chromatogram of a blank injection run between the BiTE and parent mAb showing no evidence of carryover.
Figure 2: Column Shedding and Carryover Analysis. No shedding or carryover was observed via MS total ion chromatogram.
Conclusion
The TSKgel UP-SW3000, 2 μm SEC column can be used as a platform method for bispecific antibody accurate mass determination using SEC-MS. An MS-compatible mobile phase under nondenaturing condition was successfully used with the TSKgel UP-SW3000 column. No signs of particle shedding or sample carryover, which may interfere with MS signal response, were noted with the TSKgel UP-SW3000 column.
*SEC-MS analysis was performed by the Wistar Proteomics and Metabolomics Facility (Philadelphia, PA). TSKgel and Tosoh Bioscience are registered trademarks of Tosoh Corporation, BiTE is a registered trademark of Amgen Inc. Corporation, Nexera is a registered trademark of Shimadzu Corporation, Q Exactive is a trademark of Thermo Fisher Scientific Inc.

Tosoh Bioscience LLC
3604 Horizon Drive, Suite 100, King of Prussia, PA 19406
tel. (484) 805-1219, fax (610) 272-3028
Website: www.tosohbioscience.com

Best of the Week: Flow Imaging Microscopy, Electric Vehicles, Air Pollution in India
April 11th 2025Top articles published this week include an interview about flow imaging microscopy, a news story on fiber optics and electric vehicles, and a recap of a study that explored the use of micro-particle-induced X-ray emission (micro-PIXE) spectroscopy to trace urban and indoor air pollution sources in India.