Analysis of Desalination Discharge Brines for Elements of Environmental and Economic Importance with ICP-OES
Desalination has been growing rapidly globally to meet the potable water demands in areas where access to fresh water is limited. The byproduct of desalination is a brine that has a salinity approximately two times higher than seawater and is usually discharged back to the ocean, where it can have a negative environmental impact, especially if harmful elements were picked up during the desalination process. However, this brine has recently begun to be viewed as a resource where elements of economic and industrial importance can be recovered. Both these applications rely on the accurate analysis of various elements in the brine, which can be accomplished with inductively coupled plasma–optical emission spectroscopy (ICP-OES), given its high matrix tolerance and flexibility. This work demonstrates the accurate analysis of desalination discharge brines for elements of both environmental and economic importance using ICP-OES.
With climate change, access to fresh water is decreasing in many areas around the world. Combined with the continuing rise in global population, a shortage of potable water is imminent unless other sources of fresh water can be found. One way to increase amounts of potable water is through desalination of seawater, a technique that has been rapidly growing in importance. In 2019, there were approximately 16,000 desalination plants operating globally (1), and that number has increased yearly as more desalination plants come online.
The primary method of desalination is through reverse osmosis, a process that uses high pressure to force seawater through a membrane that separates the minerals from the water, resulting in both fresh potable water and a brine that contains the minerals removed from the seawater. The salinity of these brines is generally twice the salinity of seawater (≈6% dissolved solids) and is usually pumped back into the ocean, although other treatments are also used occasionally (2). Over 142 million cubic meters of desalination brine are returned to the ocean globally on a daily basis (1).
With so much desalination brine being returned to the ocean, brine must be monitored for elements that may have been picked up during the desalination process and can have a negative impact on the aquatic environment. However, there are no international standards or regulations for monitoring elements in desalination discharge brines returned to the ocean: elements and regulated levels vary widely and are set on a country or regional basis (3–6).
However, rather than being returned directly to the ocean, these discharge brines can also be used as a source of elements of economic and industrial importance (7). A variety of different elements are recovered from desalination brines where it can be more economically feasible than mining. For example, with the prevalence of battery technology, increasing amounts of lithium are required. Currently, lithium is mined and recovered from salar brines but can also be recovered from desalination brines. Rubidium and cesium are other rare elements that are difficult to mine and can be extracted from desalination brines (8).
An important aspect in recovering elements from brines is measuring their concentrations in the brine to determine the quantity present and whether it is economically beneficial to recover the metals. Likewise, the remaining brine after the recovery step should be analyzed to determine the recovery efficiency.
The analysis of a variety of elements in desalination brines can be accomplished by inductively coupled plasma–optical emission spectroscopy (ICP-OES) with minimal sample preparation and no dilution, given that ICP-OES has a high tolerance of dissolved solids. This work explores the analysis of both environmentally and economically important elements in desalination discharge brines.
Experimental
Materials and Methods
Samples and Sample Preparation
Six desalination discharge brines were acidified to 1% nitric acid (v/v) and analyzed directly; no further sample preparation was performed. All measurements were made against external calibration standards prepared in 6% sodium chloride to mimic the dissolved solids content of the brines. The actual dissolved solids composition of the brines was not determined, but 6% sodium chloride proved to be a good match.
Instrumental Conditions
Two different instruments were used for these analyses: the PerkinElmer Avio 550 Max and the Avio 220 Max ICP-OES systems. Given the larger number of analytes, the Avio 550 Max was used for analyzing environmentally important elements, whereas the extended wavelength range of the Avio 220 Max allowed for the use of longer wavelengths, and increased sensitivity and flexibility, making it the more appropriate option for determining economically important elements. Tables I and II list the instrumental operating parameters and elements, along with their analytical wavelengths and plasma views, respectively. Because the matrix was the same on both systems, similar operating conditions were used. The main difference was the nebulizer flow. Because of the low ionization potential of lithium, rubidium, and cesium, a higher nebulizer flow was used on the Avio 220 Max to increase sensitivity. On both systems, a SeaSpray nebulizer was used in conjunction with an argon humidifier to maximize robustness. Because all analyses were done with a quartz torch, a plasma gas flow or 12 L/min was used to reduce devitrification of the torch. A ceramic torch could also be used, along with a lower plasma gas flow, although the higher plasma flow benefited the analyses performed on the Avio 220 Max. The higher-than-normal auxiliary flow was used to push the plasma farther above the injector, thus minimizing matrix deposition and potential clogging of the injector. A sample uptake rate of 0.5 mL/min was found to provide increased signal-to-background ratios because the plasma loading and matrix effects in the plasma were reduced.
Results and Discussion
Analysis of Environmentally Important Elements
When analyzing the six brine samples, only five elements were detected; all other elements were undetected. Figure 1 shows the concentrations of the five elements found in the six brine samples, along with their regulated levels in four different regions. Boron is above the Singapore limits in all six brines, whereas copper exceeds all the regulated levels in brine 2 and is higher than the Singapore limits in brine 6. Interestingly, copper was not detected in brine 5, and manganese was not detected in brines 5 or 6.
FIGURE 1: Elements detected in six desalination discharge brines, along with regulated limits in four different areas.
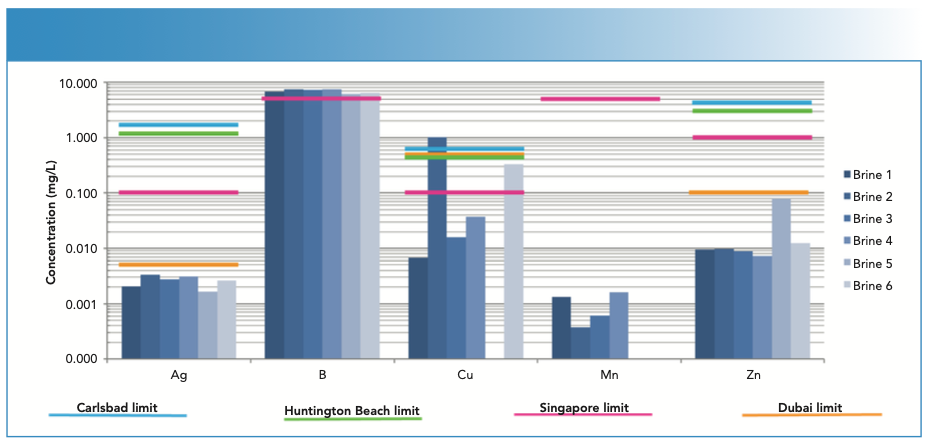
Not all elements are regulated in all regions; boron and manganese are only regulated in Singapore. Also, the regulated concentrations can vary widely among regions. For example, the regulated levels of silver vary by over two orders of magnitude. Given this wide range of regulated concentrations, the wide dynamic range of ICP-OES makes it the ideal technique to perform these analyses.
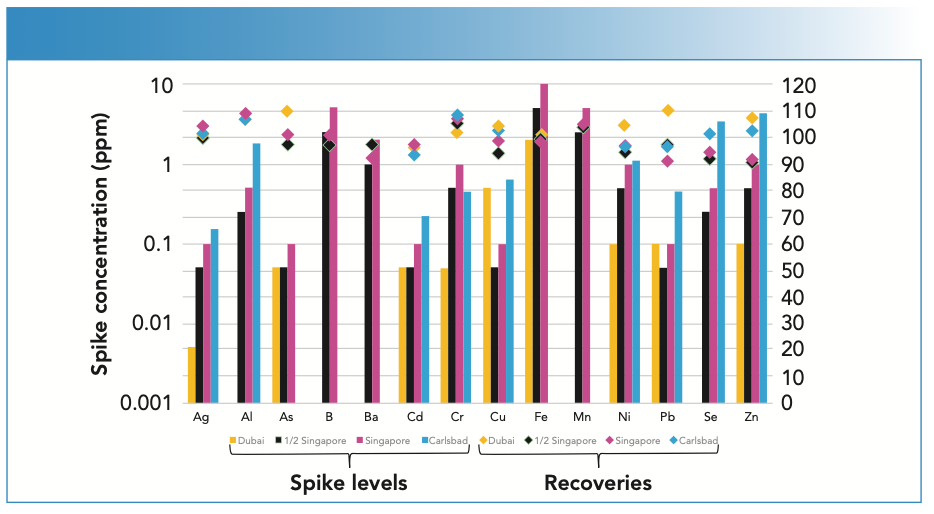
To determine the accuracy of the analyses, one of the brine solutions was spiked with all analytes at four different concentration ranges, with low representing the Dubai and half the Singapore limits, medium representing the Singapore limits, and high representing the Carlsbad limits.
As shown in Figure 2, all elements at all concentrations recovered within 10% of their true values, demonstrating the accuracy and breadth of the methodology.
FIGURE 2: Elemental recoveries of analytes at four different spike levels.
Analysis of Economically Important Elements
Cesium, lithium, and rubidium are difficult to accurately measure by ICP-OES because they emit only as atom lines, yet have low ionization potentials (3.9, 5.3, and 4.1 eV, respectively), which means that most of the signal is lost because most of the atoms in the plasma are ionized. Therefore, they are not available to emit as atoms. In addition, these elements also suffer from the easily ionizable element (EIE) effect, which means that the same concentration of these elements produce different signal levels depending on the concentration of the matrix. There are several ways to minimize the EIE effect, with the most prevalent being matrix-matching the calibration standards to the sample and viewing the plasma in radial mode at a lower plasma viewing height before the atoms are ionized. However, radial view can also result in lower signal intensity, which may be an issue when measuring low concentrations.
During method development, it was found that calibration in 6% sodium chloride minimized the EIE effect. Because of the low intensity of cesium and rubidium, these elements had to be measured in axial mode, while lithium, which has much greater sensitivity, was measured in radial mode. As shown in Figure 3, both lithium and rubidium were detected in all the brines between 0.2–0.4 ppm (lithium) and 0.1–0.2 ppm (rubidium). However, cesium was not detected in any of the samples.
FIGURE 3: Concentrations of lithium and rubidium in six desalination brines (blue; cesium was not detected) and detection limits (orange) measured in brine 5.
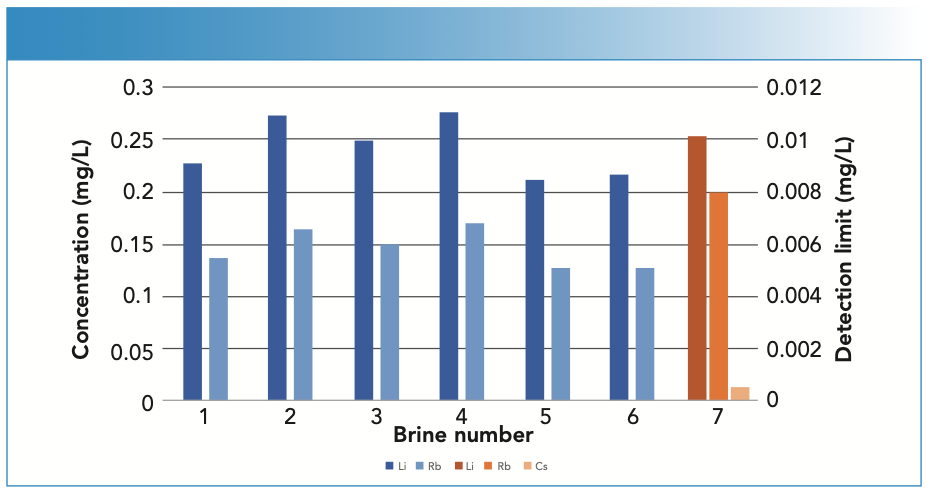
Detection limits were determined in brine 5 as three times the standard deviation of ten measurements made against the calibration curve. As shown in Figure 3, the lithium and rubidium detection limits are approximately an order of magnitude lower than the concentrations measured in the brines. The measured cesium detection limit is 0.0005 ppm, signifying that if cesium is present in the brines, it is below this concentration.
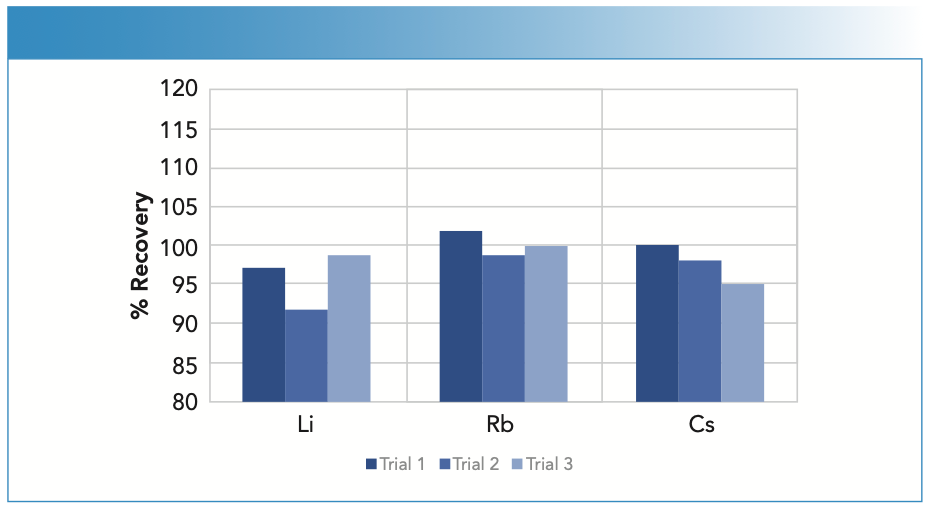
The accuracy of the methodology was determined by spiking one of the brine solutions with 0.04 ppb of cesium and 0.2 ppm of lithium and rubidium and measuring the recoveries three times. Although lithium and rubidium could be read at lower concentrations, they were spiked at 0.2 ppm because of their presence in the brine. As shown in Figure 4, all recoveries are within 10% of their true values, demonstrating the accuracy of the methodology.
FIGURE 4: Spike recoveries for 0.04 ppm cesium and 0.2 ppm lithium and rubidium in a desalination discharge brine.
Conclusion
This work has demonstrated the ability of ICP-OES to accurately measure elements in desalination discharge brines of both environmental and economic importance. Environmentally important elements are not globally regulated in desalination discharge brines and vary widely from region to region, yet ICP-OES provides accurate measurements over a wide concentration range. Likewise, these discharge brines can also be an economically viable source of a variety of elements, and this work has demonstrated ICP-OES can accurately and reliably measure cesium, lithium, and rubidium, three of the more challenging elements to measure. With the growing importance and capacity of desalination globally, ICP-OES can serve as the analytical technique to both help protect the oceans and turn a byproduct into a resource.
References
(1) United Nations University, UN Warns of Rising Levels of Toxic Brine as Desalination Plants Meet Growing Water Needs (2019). https://unu.edu/media-relations/releases/un-warns-of-rising-levels-of-toxic-brine.html (accessed September 2022).
(2) R. Ketai, T.Y. Shen, I. Jafari, S. Masudy-Panah, and M.H.D.A. Farahani, in Desalination Challenges and Opportunities, M.H.D.A. Farahani, V. Vatanpour, and A.H. Taheri, Eds. (IntechOpen, London, United Kingdom, 2020). DOI: 10.5771/intechopen.92661
(3) Singapore National Environment Agency, Allowable Limits for Trade Effluent Discharge to Watercourse or Controlled Watercourse (2022). https://www.nea.gov.sg/our-services/pollution-control/water-quality/allowable-limits-for-trade-effluent-discharge-to-watercourse-or-controlled-watercourse (accessed September 2022).
(4) Dubai Electricity and Water Authority, Discharge Limits to Marine Environment (2021). https://www.trakhees.ae/en/ehs/env/Documents/Guidelines/Guideline%20ID-EN-G02,%20Water%20Environment,%20Rev.%2000,%20Jan18.pdf (accessed September 2022).
(5) “Waste Discharge Requirements for Poseidon Resources (Surfside) L.L.C. Huntington Beach Desalination Facility Orange County”, Order R8-2021-0011, NPDSE No. CA8000403, California Regional Water Quality Control Board, Santa Ana Region.
(6) “Waste Discharge Requirements for the Poseidon Resources (Channelside) LP Claude “Bud” Lewis Carlsbad Desalination Plant Discharge to the Pacific Ocean”, Order R9-2019-0003, NPDSE No. CA 019223, California Regional Water Quality Control Board, San Diego Region.
(7) D. Frank, Mining for the Future (2022). https://www.aweimagazine.com/article/mining-for-the-future/ (accessed September 2022).
(8) W.S. Chen, C.H. Lee, H.W. Tien, Y.J. Chen, and Y.A. Chen, Metals 10, 607–622 (2020). DOI: 10.3390/met10050607
Ken Neubauer is with PerkinElmer, Inc. Direct correspondence to: Kenneth.Neubauer@perkinelmer.com ●
Microplastics Widespread on Catalan Beaches, Study Finds
March 28th 2025In a recent study published in Marine Pollution Bulletin, a team of researchers from several Spain and Portugal universities and institutions (Rovira i Virgili University, Universitat de Barcelona, University of Porto, and Institut d'Investigació Sanitaria Pere Virgili (IISPV) assessed microplastic (MP) contamination along the Mediterranean coastline.
