Applying LC with Low-Resolution MS/ MS and Subsequent Library Search for Reliable Compound Identification in Systematic Toxicological Analysis
Special Issues
Systematic toxicological analysis is an important step in medicolegal investigations of death, poisoning, and drug use. The primary goal is the detection and confirmation of potentially toxic compounds in evidence. This article describes a workflow using nontargeted liquid chromatography–tandem mass spectrometry (LC–MS/MS) for reliable compound identification.
Systematic toxicological analysis (STA) is an important step in medicolegal investigations of death, poisoning, and drug use. The primary goal is the detection and confirmation of potentially toxic compounds in evidence. This article describes a workflow using nontargeted liquid chromatography–tandem mass spectrometry (LC–MS/MS) for reliable compound identification. Tandem mass spectrometry was performed on a low-resolution quadrupole-quadrupole-linear ion trap instrument. Acquired spectra were submitted to automated library search, and positive matches were verified by expert reviewing. After validation, the nontargeted LC–MS/MS technique was integrated in the STA service provided by our laboratory.
Systematic toxicological analysis (STA) is an important step in medicolegal investigations of death, poisoning, and drug use and can be done to various kinds of samples. The most frequently used biological samples are urine, blood, and plasma. In the field of post-mortem toxicology, additional specimens such as different tissues, gastric content, bile, and hair samples are analyzed. The samples are usually processed with generic extraction procedures, including liquid–liquid extraction and solid-phase extraction (SPE). As urine samples may contain phase II metabolites of drugs and poisons, a pretreatment step involving hydrolytic cleavage of the conjugate bond is commonly performed for this kind of sample.
Mass spectrometry (MS) hyphenated with a variety of separation techniques is the most important analytical technique applied to detect and confirm exogenous compounds present in biological samples. In particular, gas chromatography–mass spectrometry (GC–MS) is considered to be the gold standard for STA (1). This technique offers reliable identification of compounds because of the use of well-established mass spectral libraries. However, as one single mass spectrometric method is not able to cover the entire chemical space of forensic toxicological interest, GC–MS is usually complemented by liquid chromatography–mass spectrometry (LC–MS) techniques (2–7). Compound identification with LC–MS usually involves accurate molecular mass measurements (8-14), multiple reaction monitoring (MRM) (15–19), and acquisition of tandem mass spectra with subsequent library search (3–7, 20–37).
MRM is part of targeted assays. Such approaches provide very low limits of detection and quantitative information, but the number of compounds included is limited to a few hundred species only (21,23).
Nontargeted procedures usually use full-scan MS as the survey scan and combine it with data-dependent tandem mass spectrometry (MS/MS) scans (26). The automated switch from MS to MS/MS and back again is controlled by software. This data-dependent acquisition (DDA) enables recording of MS/MS spectra of almost all compounds detected in MS. An inherent limitation of DDA is the exclusion of low-abundant species particularly in complex samples because of the use of intensity thresholds and restriction of the number of ions submitted to fragmentation. To further increase detection sensitivity, data-independent acquisition (DIA) strategies were presented (29,34,35,37,38). In DIA, principally all ions detected in MS are submitted to MS/MS. One limitation of DIA is that this technique seems to work efficiently only on high-resolution instruments, such as quadrupole-quadrupole-time of flight (QqTOF), or orbital ion trap. Fourier transform ion cyclotron resonance (FT-ICR) instruments also belong to this class of instruments. They are, however, not common for this type of application. Furthermore, the full potential of DIA can only be exploited with data deconvolution algorithms that enable fast and efficient extraction of compound-specific tandem mass spectra. Irrespective of the kind of acquisition control employed, by subsequent matching of the obtained MS/MS spectra to sufficiently large collections of high-quality reference spectra, such as MassBank (39), NIST (40), or the “Wiley Registry of Tandem Mass Spectral Data, MSforID” (41), unequivocal identification of thousands of compounds is feasible.
Initially, nontargeted LC–MS/MS methods with DDA were developed on low-resolution instruments (4,20,22,25,42). Currently, however, high-resolution QqTOF as well as orbital ion trap instruments are preferentially used for that purpose (8–10). The mass accuracy of these instruments is considered to represent a prime requisite for reliable compound identification. For instance, we have recently shown that a QqTOF instrument with DDA of MS/MS spectra and subsequent library search for STA of 65 authentic casework samples produced only 1.0% false positive results (3). In contrast, STA procedures involving automated library search with low-resolution data were reported to produce a significantly higher number of false positive results (31,43).
Wissenbach and colleagues tested the performance and transferability of their tandem mass spectral library (31). The group built a linear ion trap (LIT) library of MSn spectra covering 4500 compounds. They analyzed 100 authentic urine samples with two LC–MS/MS-based screening strategies: (1) a LC–MSn approach using a LIT instrument and (2) a LC–MS/MS approach using a quadrupole-quadrupole-linear ion trap (QqLIT) instrument. To improve performance of the library for QqLIT spectra, MSn library spectra were merged. Automated library search was accomplished with “X-Rank” (44). The MSn data produced 3362 positive matches; 1111 (33%) were found to be false positives by expert reviewing. The QqLIT data produced 3091 positive matches, and 1491 (48%) were found to be false positives.
Lynch and colleagues applied two different LC–MS/MS-based screening approaches for the analysis of 48 authentic urine samples (43). For both screening assays low-resolution instruments were used to generate MS/MS data. Identifications obtained by automated library search were checked by expert reviewing. The authors reported false positive rates of up to 37% and false negative rates of up to 49%. In the majority of cases, low quality spectra that were not efficiently handled by automated library search were identified as causes of incorrect matches.
Limited reliability of automated library search increases time and effort spent for expert reviewing of STA results. Accordingly, data processing is often regarded as a bottleneck. To overcome this limitation, advanced search algorithms showing increased tolerance against changes of fragment ion intensities are required. As such software tools have become available recently (44–47), the development of nontargeted LC–MS/MS methods with DDA and automated library search employing low-resolution instruments should be possible.
In this article, we present a nontargeted LC–MS/MS screening approach for STA of blood, plasma, and urine samples that uses low-resolution QqLIT for MS/MS spectra generation. The instrument is operated in positive electrospray ionization (ESI) mode with DDA. Sample processing involves a generic SPE method. Cleavage of the phase II metabolites in the urine samples is enabled by enzymatic hydrolysis. Chromatographic separations are accomplished with reversed-phase LC. The acquired MS/MS spectra were submitted to automated library search in the “Wiley Registry of Tandem Mass Spectral Data, MSforID.” Putative correct positive matches are reviewed by an expert. The nontargeted LC–MS/MS method has been validated. Results of experiments assessing its selectivity, detection capabilities, and reliability of identification (sensitivity and specificity) are discussed. Finally, the usefulness of integrating the LC–MS/MS screening approach in our GC–MS-based analysis service will be demonstrated by giving an overview on the screening results obtained from 919 authentic samples submitted to STA from 2012 to 2014.
Materials and Methods
Chemicals and Samples
Water, methanol, sodium hydroxide, potassium hydroxide (all reagent grade), acetic acid (HOAc), heptafluorobutyric acid (HFBA), hydrochloric acid (37%), potassium dihydrogen phosphate (all puriss p.a.), ammonium hydroxide (25%), and ethyl acetate (all analytical reagent grade) were obtained from Sigma Aldrich. Phosphoric acid (86–87%, analytical reagent grade) was purchased from Scharlau. Propan-2-ol and dichloromethane (all analytical reagent grade) were supplied by Fisher Scientific. Acetic anhydride (extra pure) was obtained from Merck. Pyridine (AnalaR Normapur) was purchased from VWR. Bunitrolol hydrochloride was obtained from Chemicals International.
A 0.1 M phosphate buffer (pH 6.0) was prepared by adding appropriate amounts of potassium hydroxide to an aqueous solution of potassium dihydrogen phosphate.
Drug standards that were used for the preparation of spiked plasma samples were taken from the laboratory’s collection. The standards were either obtained from commercial suppliers (Lipomed; LGC Promochem) or from the manufacturers of the marketed drugs.
For enzymatic hydrolysis of urine samples, a mixture of β-glucuronidase and arylsulfatase (Helix pomatia, Roche Diagnostics) was used.
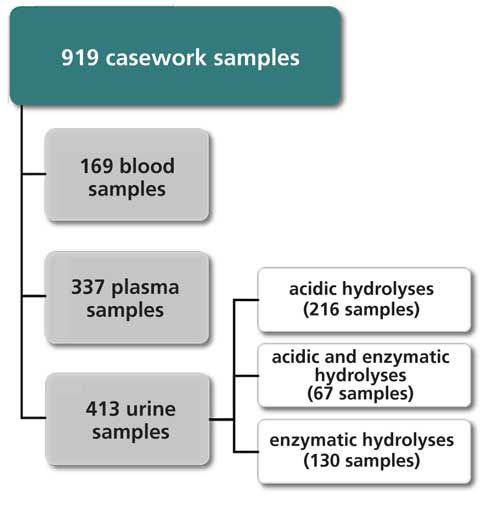
Blank urine samples were provided from healthy volunteers. Blank plasma samples were kindly donated by the blood bank of the Medical University of Innsbruck (Austria). The set of authentic casework samples consisted of 919 samples (blood, plasma, and urine) submitted to forensic toxicological examination (Figure 1). Sample collection was accomplished by medical doctors according to guidelines published by the “Österreichische Gesellschaft für Gerichtliche Medizin” (http://oeggm.com/oeggm-qualittssicherung.html). All samples were stored at -20 °C prior to analysis.
Figure 1: Types of authentic casework samples analyzed in this study.
The STA Workflow
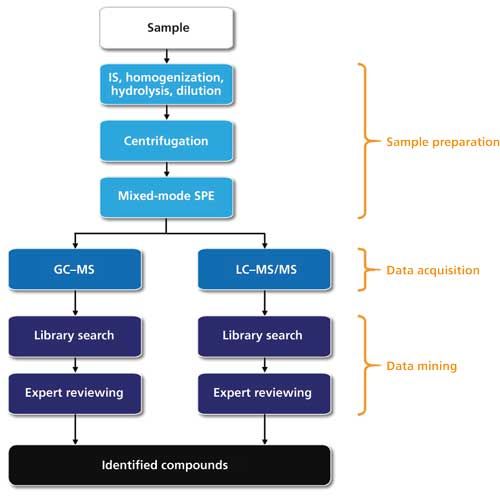
The setup of the developed STA workflow is shown in Figure 2. It involves sample preparation, data acquisition, and data mining steps.
Figure 2: Workflow developed for STA with parallel GC–MS and LC–MS/MS analysis.
Sample Preparation
Samples were processed with sample preparation workflows established in our laboratory for STA of blood, plasma, and urine (3).
Blood or plasma samples (2.0 mL) were mixed with 40 µL of an aqueous solution of the internal standard (IS, fencamfamine, 60 µg/mL) and diluted with 4 mL of distilled water as well as 4 mL of 0.1 M phosphate buffer (pH 6.0).
Acidic and/or enzymatic hydrolysis was used to cleave phase II metabolites in urine samples. For acidic hydrolysis, 10.0 mL of urine was split in two 5.0-mL aliquots. One aliquot was submitted to hydrolysis. Urine (5.0 mL) was mixed with 2.0 mL of 6 M hydrochloric acid. A 40 µL measure of an aqueous solution of fencamfamine (60 µg/mL) was added as internal standard. The sample was incubated in a microwave oven (450 W) for 60 s. After cooling, the samples were adjusted to pH 6 with sodium hydroxide and 4.0 mL of 0.1 M phosphate buffer (pH 6.0). The pH was checked with indicator paper. Finally, the hydrolyzed sample was mixed with the corresponding neat urine sample. For enzymatic hydrolysis, 10.0 mL of urine were mixed with 4.0 mL of 0.1 M phosphate buffer (pH 6.0), 40 µL of an aqueous solution of fencamfamine (60 µg/mL), and 200 µL of the enzyme solution (1.1 units β-glucuronidase and 0.52 units arylsulfatase, Roche Diagnostics). The sample was incubated for 16 h at 40 °C.
Prior to SPE, all preprocessed samples were centrifuged for 5 min at 4500g; only the supernatants were used. SPE was performed on Spe-ed Scan ABN columns (200 mg/3 mL, Applied Separations). Before application of the sample, the SPE column was conditioned with 2 mL methanol and 2 mL 0.1 M phosphate buffer (pH 6.0). Loading of sample was accomplished at a flow rate of 1.0–1.5 mL/min. Next, the column was washed with 3 mL of distilled water, 1 mL 1.0 M HOAc, and 1 mL of an aqueous methanol solution (5%, v/v), centrifuged for 5 min (4500g), and dried with a nitrogen stream. Elution was performed with 2.0 mL of a mixture of dichloromethane, propan-2-ol, and ammonium hydroxide (80/20/8, v/v/v). The eluate was split in two 1.0-mL aliquots. The aliquots were evaporated to dryness at 60 °C under a gentle stream of nitrogen. The residue of one aliquot was reconstituted in 50 µL of aqueous 0.5% HOAc/0.005% HFBA solution containing 5% methanol (v/v) and submitted to LC–MS/MS analysis. The residue of the second aliquot was reconstituted in 50 µL of ethyl acetate and submitted to GC–MS analysis. The GC–MS sample was analyzed in the native form and after derivatization. After evaporation to dryness at 60 °C under a gentle stream of nitrogen, 50 µL pyridine and 100 µL acetic anhydride were added. Derivatization was performed at 60 °C for 45 min. After evaporation of excess of derivatizing reagent, the residue was reconstituted in 50 µL ethyl acetate.
LC–MS/MS
The LC system consisted of a K-1001 pump (Knauer) and a HTS PAL autosampler (CTC Analytics) equipped with a 20-µL injection loop. The injected sample volume was 10 µL. Chromatographic separations were accomplished on a 100 x 2 mm, 5-µm, 100 Å Eurospher C18 column (Knauer) using a 10-min gradient of 5–100% methanol in aqueous 0.5% HOAc/0.005% HFBA solution. The column temperature was held at 50°C with a column oven (Thermotechnic Products GmbH). The flow rate was set to 100 µL/min. The column outlet was directly connected to the ESI source.
ESI-MS was performed in the positive ion mode using a QqLIT instrument (3200 Q Trap, Sciex) equipped with a TurboIonSpray source (Sciex). Optimization of instrumental parameters was performed by infusion of 5.0 mg/L bunitrolol dissolved in aqueous 0.5% HOAc/0.005% HFBA solution containing 5% methanol (v/v) at a flow rate of 20 μL/min. The spray voltage was set to 5.5 kV. Gas flows of 45 arbitrary units for the nebulizer gas and 25 arbitrary units for the turbo gas were used. The temperature of the turbo gas was adjusted to 500 °C.
The mass spectrometer was operated under DDA control. A duty cycle in DDA mode included a Q3 MS scan followed by enhanced resolution (ER) scans and enhanced product ion (EPI) scans on the three most abundant precursor ions (processed in reverse order of abundance). The intensity threshold for triggering MS/MS experiments was 20,000 counts. Isolation of precursor ions was accomplished with Q1 set to unit resolution. The collision gas flow (N2) was set to “high.” For each precursor, MS/MS spectra were generated at a collision energy of 30 eV with a collision energy spread of 10 eV. Mass spectra were acquired from 135 to 700 m/z, and MS/MS spectra from 50 to 700 m/z. Accumulation times were set to 0.5 s for MS scans. The scan rate for ER scans and EPI scans was 1000 Da/s. Thus, the overall scan time per duty cycle was approximately 4.5 s. Spectra were recorded on a personal computer with Analyst software 1.5 (Sciex).
Library Search
Raw data files (.wiff) were converted to Mascot Generic Format (.mgf) files with the Sciex MS Data Converter (version 1.3 beta). Next, the MS/MS spectra part of the .mgf file were extracted with a program written in ActivePerl 5.6.1 (Active State Corporation). Thus, all MS/MS spectra were available as plain text (ASCII) files containing peak list information, and they were used as input for library search.
The “Wiley Registry of Tandem Mass Spectral Data, MSforID” (Wiley) served as the reference library (41). The library was developed on a QqTOF instrument (Qstar XL, Sciex) using ESI in positive and negative ion mode. A detailed description of the instrumental parameters applied can be found elsewhere (47,48). At the current stage of development, the library contains 12,122 spectra of 1208 compounds.
The principles of the MSforID library search program have been described elsewhere (46,47,49). The search algorithm determines similarity between a sample spectrum and library spectra. The degree of similarity is expressed by two values: the “average match probability” (mp) and the “relative average match probability” (ramp), respectively. High compound-specific amp- and ramp-values indicate high similarity between the unknown and the reference compound. The substance with the highest amp- and ramp-values is considered to represent the unknown compound.
Automated MSforID search was performed with a program written in Pascal using Delphi 6 for Windows (Borland Software Corporation; now Embarcadero Technologies, Inc.) using the following search parameters: m/z tolerance of ±0.1, intensity cut-off factor of 0.01–0.05. The following criteria were used to classify obtained search results as putatively correct positive results: precursor ion mass tolerance of ±0.10, amp >10.0, and ramp >40.0. The accuracy of each putatively positive match was checked by expert reviewing.
GC–MS
The GC–MS system consisted of a HP7890 gas chromatograph with a HP5975C inert XL mass-selective detector (Agilent Technologies). A 30 m x 0.25 mm, 0.25-µm DB-XLB column (J&W Scientific) was used for chromatographic separation. Carrier gas was helium with a flow rate of 1.0 mL/min. Injection volume was 2 µL (splitless) and injection temperature was 250 °C. The temperature program was as follows: 50 °C, hold 1 min; increase to 150 °C with 25 °C/min, to 320 °C with 10 °C/min, hold for 8 min and to 330 °C in 20 °C/min, hold for 7.5 min. MS was performed in electron impact mode (70 eV) scanning from 50 to 600 m/z. Mass spectral data were recorded on a personal computer with the HP MS ChemStation software G1034C version D01.00 (Agilent Technologies) including the Maurer/Pfleger/Weber 2011 mass spectral library (Wiley) for compound identification.
Performance Evaluation
Evaluation of the performance of the nontargeted LC–MS/MS method developed for STA of urine, blood, and plasma samples was accomplished according to published recommendations for the validation of qualitative methods (50–52). The following performance parameters were examined: selectivity, detection capability, and reliability of identification (sensitivity and specificity).
Selectivity of the LC–MS/MS procedure and specificity of automated library search were evaluated by analyzing nine blank plasma samples and eight blank urine samples. All extracts were analyzed twice. The origin and plausibility of correct positive results was evaluated. Furthermore, the rate of false positive matches was determined to estimate specificity of the screening assay.
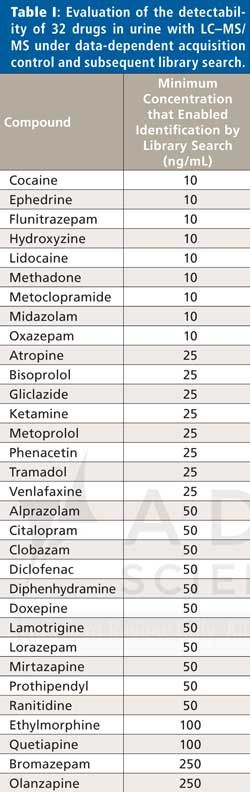
For evaluation of the detection capability of the LC–MS/MS method and the sensitivity of automated library search, spiked plasma and urine samples were analyzed. Blank plasma samples were fortified with 66 compounds at concentrations equal to the individual lower limits of the therapeutic blood/plasma ranges. A list of the compounds studied is provided in the Electronic Supplementary Material (ESM) Table S1 at: www.chromatographyonline.com/applying-lc-low-resolution-msms-and-subsequent-library-search-reliable-compound-identification-syste. Therapeutic ranges were extracted from the literature (53). Blank urine samples were spiked with 32 compounds at six different concentration levels (10 ng/mL, 25 ng/mL, 50 ng/mL, 100 ng/mL, 250 ng/mL, and 500 ng/mL). A list of the compounds studied can be found in Table I. The samples were analyzed, and the minimum concentrations enabling identification of the compounds by automated library search were determined.
A further proof of the reliability of the developed LC–MS/MS screening approach was obtained by analyzing 919 authentic casework samples (Figure 1). For benchmarking, GC–MS was used as a reference method. To simplify the comparison, multiple matches to a single reference compound were handled as one match only. Furthermore, all matches indicating intake of a certain drug compound (that is, the drug itself, metabolites as well as artifacts) were combined to one entry in the hit list (= “identified compound”). The following example may illustrate this modus operandi: if LC–MS/MS screening of a sample produces five MS/MS spectra that are matched to cocaine and several more spectra that are matched to benzoylecgonine and methylecgonine, then the five cocaine matches as well as the benzoylecgonine and methylecgonine matches are combined to the hit list entry “cocaine.” Endogenous compounds (for example, adenine) as well as commonly observed nutritional compounds (caffeine and nicotine) were not included in the statistical evaluation of the results obtained.
Results and Discussion
Evaluation of the Performance of the LC–MS/MS Method
Method validation is the process of sufficiently developing a picture of the performance of a method to demonstrate that it is fit for an intended purpose. To demonstrate the usefulness of the LC–MS/MS method for STA, the following parameters were studied: selectivity, detection capability, and reliability of identification (sensitivity and specificity).
Selectivity of the developed LC–MS/MS method and specificity of automated library search were tested by analyzing nine plasma and eight urine samples donated from volunteers who did not consume any drug. All extracts were screened twice by nontargeted LC–MS/MS.
The plasma samples produced 11,306 spectra; 136 spectra gave rise to correct positive identifications, nine matches were found to be incorrect. Thus, the false positive rate of automated library search was 0.08% only.
The urine samples produced 11,687 spectra; 237 spectra gave rise to correct positive identifications, 56 matches were found to be incorrect. Thus, the false positive rate of automated library search was 0.48% only.
The compounds correctly identified included the internal standard, nutritional compounds (caffeine, piperine), and endogenous compounds (adenine).
Automated library search was found to be very specific (>99%). The observed false positive rates were comparable to values previously reported for high-resolution instruments (2,35). As the low number of false positive matches produced by software were sorted out by expert reviewing, the LC–MS/MS procedure clearly passed the selectivity test.
Detection capabilities of the developed LC–MS/MS method and sensitivity of automated library search were tested by analyzing blank plasma and urine samples fortified with reference standards. For plasma, the lower end of the therapeutic range was defined as the minimum required performance limit at which a compound should be reliably detected and confirmed (3). To test detection sensitivity, blank plasma samples were spiked with 66 compounds present at concentrations equal to the individual lower limits of their therapeutic ranges and analyzed by LC–MS/MS. The obtained results are summarized in Figure 3. Down to a minimum therapeutic concentration of 20 ng/mL, the developed LC–MS/MS assay represents an efficient screening tool. More than 80% of all compounds were detected and identified. There were only four compounds with a minimum therapeutic concentration of ≥100 ng/mL that were not detected. For 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), in-source fragmentation might have negatively affected detectability. Fluoxetine produced an inconclusive fragmentation pattern at the collision energy settings employed, and diclofenac contained an acidic group that obviously had a negative effect on ionization efficiency.
Figure 3: Impact of the plasma concentration on the detectability of 66 representative compounds with LC–MS/MS.
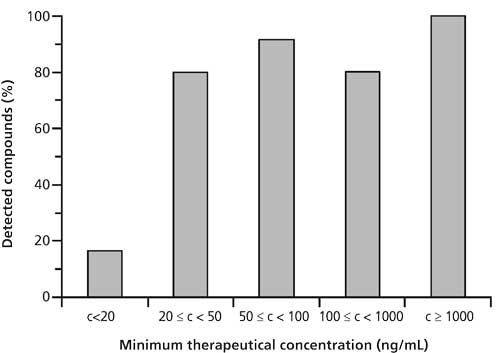
In terms of the detection limits, the LC-MS/MS method was less efficient than our previously presented QqTOF method (3). Nevertheless, as with the detection sensitivity provided the majority of compounds were detectable and identifiable at therapeutic and thus even at the higher toxic and comatose-fatal blood-plasma concentrations (53), we regard the herein presented LC–MS/MS method sufficiently sensitive for application in STA.
To characterize the detection capabilities of the LC–MS/MS method for urine samples, limits of detection (LOD) values were determined for 32 compounds. Blank urine samples were spiked with test compounds at six different concentration levels (10 ng/mL, 25 ng/mL, 50 ng/mL, 100 ng/mL, 250 ng/mL, and 500 ng/mL). The samples were analyzed and the minimum concentrations enabling identification of the compounds by automated library search were determined (Table I). The majority of compounds were detectable at concentrations ≤50 ng/mL (87.5%). Ethylmorphine, quetiapine, bromazepam, and olanzapine showed higher LOD values.
The detection capabilities of the presented workflow are sufficient to enable application in STA. We believe, however, that more modern instrumentation will provide lower limits of detection, which would significantly improve detection capabilities of the nontargeted LC–MS/MS workflow described.
The tandem mass spectral data obtained from testing the detection capabilities of the LC–MS/MS method were further used to characterize sensitivity of automated library search. Sensitivity (also called the true positive rate or the recall rate) measures the proportion of actual positives, which are correctly identified as such. We defined a positive as a reference compound spiked in a sample that triggered the acquisition of one or more MS/MS spectra in DDA mode. A true positive identification was obtained if one of the spectra representing a positive returned a classified match (precursor ion mass tolerance of ±0.10, amp >10.0, and ramp >40.0) to the corresponding reference compound from an automated library search. With both sets of spiked samples, 198 positives were generated, and only two of them produced false negative results (ethylmorphine at 50 ng/mL and bromazepam at 100 ng/mL in urine). Thus, sensitivity of automated library search was found to be 99%.
Application of the STA Workflow to the Analysis of Forensic Casework Samples
After demonstrating that the developed LC–MS/MS method is fit for purpose, we decided to integrate it into the GC–MS-based STA workflow part of the forensic-toxicological analysis service offered by our laboratory (Figure 2). This was realized in June 2012. By October 2014, 919 casework samples, including ante- and post-mortem samples, were analyzed with this parallel screening approach (Figure 1).
From June 2012 to October 2014, 169 blood samples and 337 plasma samples were received by the casework unit. STA of these samples led to 629 identifications. In 345 cases (54.8%), the presence of a compound was confirmed by LC–MS/MS and GC–MS, in 139 cases by LC–MS/MS only (22.0%), and in 145 cases by GC–MS only (23.0%). As expected, parallel screening with complementary mass spectrometric methods was a competent approach to increase reliability and validity of STA. In the majority of cases, identification was based on the results obtained by two independent methods. Furthermore, some compounds were preferentially detected by one of the two methods applied. LC–MS/MS was found to be particularly useful for the detection of beta blockers and benzodiazepines. Compounds with low-ionization efficiency in positive ESI (for example, propofol, diclofenac, ibuprofen, and barbiturates), on the other hand, were preferentially detected by GC–MS.
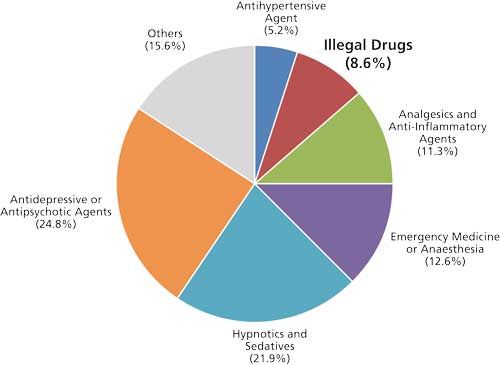
With 629 identifications obtained from the 506 blood or plasma samples, consumption of 124 different drug compounds was confirmed. An overview on important compound classes observed is provided in Figure 4. Out of the 629 identifications, 156 (24.8%) involved antidepressive or antipsychotic agents, such as citalopram, trazodone, quetiapine, mirtazapine, and prothipendyl. Other commonly observed medications belonged to the classes of hypnotics, sedatives, anesthetics, and analgesics. These kinds of drugs are commonly used in emergency medicine and critical care, and included midazolam, diazepam, lidocaine, propofol, and ketamine. Illegal drugs (morphine, methadone, cocaine, and MDMA) were identified in 8.6% of cases.
Figure 4: Important drug classes found in 169 plasma and 337 blood samples.
From June 2012 to October 2014, 413 urine samples were analyzed (Figure 1), and 1036 identifications were obtained. With this information, consumption of 165 different drug compounds was confirmed.
Processing of urine samples included a hydrolysis step to cleave phase II metabolites. In 2013, the hydrolysis protocol was changed from acidic hydrolysis to enzymatic hydrolysis with β-glucuronidase and arylsulfatase. To demonstrate the usefulness of this workflow modification, 67 urine samples were processed with both cleavage methods (Figure 1). Acidic hydrolysis enabled 153 identifications, and enzymatic treatment enabled 161 identifications. In particular, the detectability of benzodiazepines was found to be improved with the latter method.
Overall 197 urine samples were analyzed with the workflow that involved enzymatic hydrolysis (Figure 1) and 523 identifications were obtained. In 291 cases (55.6%), the presence of a compound was confirmed by LC–MS/MS and GC–MS, in 46 cases by LC–MS/MS only (15.8%), and in 186 cases by GC–MS only (28.6%).
With the 186 identifications obtained by GC–MS only, consumption of 64 different compounds was verified. There were three different reasons why LC–MS/MS was not able to detect or identify these species: (1) The compound was only included in the GC–MS library (31% of cases); (2) The compound was included in both libraries; identification was based on matches to transformation products or artifacts, which were only included in the GC–MS library (27% of cases); (3) The compound was included in both libraries; the compound was detected by GC–MS only (42% of cases).
Despite considerable success of the developed LC–MS/MS screening procedure, the limited number of compounds included in the tandem mass spectral library seems to be a shortcoming. With 1208 compounds, our library covers only part of the entire chemical space of potentially toxic compounds. Furthermore, some important drug metabolites and artifacts are missing. Both limitations are currently being addressed. We hope that these problems will be solved with a revised and extended version of the library.
A further shortcoming is the limited detection sensitivity of acidic compounds in positive ESI. This problem will be addressed by developing a nontargeted LC–MS/MS screening procedure with negative ESI.
Conclusions
STA is an integral part of the medicolegal investigation of death, poisoning, and drug use. The outcome of STA can have substantial legal and social consequences. Accordingly, the probability of a false result should be minimized by applying generic analytical techniques that enable sensitive detection and reliable identification of a large number of potentially toxic compounds. As a result of the complementarity of GC–MS and LC–MS/MS, we and others have shown that parallel screening with both methods is a competent STA strategy (2–7). With this study we provide further evidence for the usefulness of this approach.
The presented nontargeted LC–MS/MS method was developed on a low-resolution QqLIT instrument. Validation experiments clearly proved its fitness for STA of plasma, blood, and urine samples. The suitability of the developed LC–MS/MS screening approach for application in a forensic-toxicological analysis service was demonstrated by screening 919 casework samples, including ante- and post-mortem samples. STA led to >1600 identifications. More than 160 different drug compounds were detectable, and those included antidepressants, antipsychotics, hypnotics, sedatives, anaesthetics, analgesics, and illegal drugs.
It bears mentioning that our approach involved a QqLIT instrument for data acquisition and a tandem mass spectral library developed on a QqTOF instrument for identification. Nevertheless, sensitivity and specificity of an automated library search were found to be ≥99%. This observation clearly demonstrates that a database consisting of a properly designed tandem mass spectral library and a tailor-made search algorithm can be transferred between different types of instruments to enable reliable compound identification in authentic casework samples.
Despite considerable success of the developed STA procedure, there is still room for improvements. Problems related to detection sensitivity will be overcome by using more modern instrumentation and screening in negative ESI. Shortcomings of the tandem mass spectral library will be addressed by increasing its content. In particular, spectra of important drug metabolites and artifacts will be added.
Acknowledgments
We want to thank the members of our casework unit (Institute of Legal Medicine, Medical University of Innsbruck) for their assistance.
References
- H.H. Maurer, J. Mass Spectrom.41, 1399–1413 (2006).
- H. Oberacher and K. Arnhard, Bioanalysis7, 2825–2840 (2015).
- H. Oberacher, B. Schubert, K. Libiseller, and A. Schweissgut, Anal. Chim. Acta 770, 121–131 (2013).
- F.L. Sauvage, F. Saint-Marcoux, B. Duretz, D. Deporte, G. Lachatre, and P. Marquet, Clin. Chem.52, 1735–1742 (2006).
- D.M. Mueller, B. Duretz, F.A. Espourteille, and K.M. Rentsch, Anal. Bioanal. Chem. 400, 89–100 (2011).
- D.K. Wissenbach, M.R. Meyer, D. Remane, A.A. Philipp, A.A. Weber, and H.H. Maurer, Anal. Bioanal. Chem. 400, 3481–3489 (2011).
- S. Broecker, S. Herre, B. Wüst, J. Zweigenbaum, and F. Pragst, Anal. Bioanal. Chem.400, 101–117 (2011).
- M.R. Meyer, A.G. Helfer, and H.H. Maurer, Bioanalysis6, 2275–2284 (2014).
- I. Ojanpera, M. Kolmonen, and A. Pelander, Anal. Bioanal. Chem.403, 1203–1220 (2012).
- A.H. Wu, R. Gerona, P. Armenian, D. French, M. Petrie, and K.L. Lynch, Clin. Toxicol.50, 733–742 (2012).
- M. Gergov, B. Boucher, I. Ojanpera, and E. Vuori, Rapid Commun. Mass Spectrom.15, 521–526 (2001).
- A. Pelander, I. Ojanpera, S. Laks, I. Rasanen, and E. Vuori, Anal. Chem.75, 5710–5718 (2003).
- A. Polettini, R. Gottardo, J.P. Pascali, and F. Tagliaro, Anal. Chem.80, 3050–3057 (2008).
- R.I.D. Birkler, R. Telving, O. Ingemann-Hansen, A.V. Charles, M. Johannsen, and M.F. Andreasen, Forensic Sci. Int. 222, 154–161 (2012).
- D. Montenarh, M. Hopf, H.H. Maurer, P. Schmidt, and A.H. Ewald, Anal. Bioanal. Chem.406, 803–818 (2014).
- M. Di Rago, E. Saar, L.N. Rodda, S. Turfus, A. Kotsos, D. Gerostamoulos, and O.H. Drummer, Forensic Sci. Int.243, 35–43 (2014).
- D. Remane, D. Wetzel, and F.T. Peters, Anal. Bioanal. Chem.406, 4411–4424 (2014).
- M. Fisichella, L. Morini, C. Sempio, and A. Groppi, Anal. Bioanal. Chem.406, 3497–3506 (2014).
- S. Strano-Rossi, S. Odoardi, M. Fisichella, L. Anzillotti, R. Gottardo, and F. Tagliaro, J. Chromatogr. A1372, 145–156 (2014).
- F. Saint-Marcoux, G. Lachâtre, and P. Marquet, J. Am. Soc. Mass Spectrom. 14, 14–22 (2003).
- C.A. Mueller, W. Weinmann, S. Dresen, A. Schreiber, and M. Gergov, Rapid Commun. Mass Spectrom.19, 1332–1338 (2005).
- S. Dresen, M. Gergov, L. Politi, C. Halter, and W. Weinmann, Anal. Bioanal. Chem.395, 2521–2526 (2009).
- S. Dresen, N. Ferreirós, H. Gnann, R. Zimmermann, and W. Weinmann, Anal. Bioanal. Chem. 396, 2425–2434 (2010).
- F.L. Sauvage, N. Picard, F. Saint-Marcoux, J.M. Gaulier, G. Lachatre, and P. Marquet, J. Sep. Sci. 32, 3074–3083 (2009).
- F.L. Sauvage and P. Marquet, Anal. Bioanal. Chem.396, 1947 (2010).
- T.N. Decaestecker, K.M. Clauwaert, J.F. Van Bocxlaer, W.E. Lambert, E.G. Van den Eeckhout, C.H. Van Peteghem, and A.P. De Leenheer, Rapid Commun. Mass Spectrom.14, 1787–1792 (2000).
- T.N. Decaestecker, S.R. Vande Casteele, P.E. Wallemacq, C.H. Van Peteghem, D.L. Defore, and J.F. Van Bocxlaer, Anal. Chem. 76, 6365–6373 (2004).
- H.C. Liu, R.H. Liu, H.O. Ho, and D.L. Lin, Anal. Chem. 81, 9002–9011 (2009).
- H.K. Lee, C.S. Ho, Y.P. Iu, P.S. Lai, C.C. Shek, Y.C. Lo, H.B. Klinke, and M. Wood, Anal. Chim. Acta649, 80–90 (2009).
- D.K. Wissenbach, M.R. Meyer, D. Remane, A.A. Weber, and H.H. Maurer, Anal. Bioanal. Chem.400, 79–88 (2011).
- D.K. Wissenbach, M.R. Meyer, A.A. Weber, D. Remane, A.H. Ewald, F.T. Peters, and H.H. Maurer, J. Mass Spectrom.47, 66–71 (2012).
- S. Broecker, S. Herre, and F. Pragst, Forensic Sci. Int.218, 68–81 (2012).
- H.R. Lin, C.C. Liao, and T.C. Lin, Rapid Commun. Mass Spectrom.28, 2043–2053 (2014).
- A.T. Roemmelt, A.E. Steuer, M. Poetzsch, and T. Kraemer, Anal. Chem.86, 11742–11749 (2014).
- K. Arnhard, A. Gottschall, F. Pitterl, and H. Oberacher, Anal. Bioanal. Chem. 407, 405–414 (2015).
- J. Kempf, J. Traber, V. Auwarter, and L.M. Huppertz, Forensic Sci. Int.243, 84–89 (2014).
- N.S. Chindarkar, M.R. Wakefield, J.A. Stone, and R.L. Fitzgerald, Clin. Chem. 60, 1115–1125 (2014).
- J.D. Chapman, D.R. Goodlett, and C.D. Masselon, Mass Spectrom. Rev.33, 452–470 (2014).
- H. Horai, M. Arita, S. Kanaya, Y. Nihei, T. Ikeda, K. Suwa, Y. Ojima, K. Tanaka, S. Tanaka, K. Aoshima, Y. Oda, Y. Kakazu, M. Kusano, T. Tohge, F. Matsuda, Y. Sawada, M.Y. Hirai, H. Nakanishi, K. Ikeda, N. Akimoto, T. Maoka, H. Takahashi, T. Ara, N. Sakurai, H. Suzuki, D. Shibata, S. Neumann, T. Iida, K. Tanaka, K. Funatsu, F. Matsuura, T. Soga, R. Taguchi, K. Saito, and T. Nishioka, J. Mass Spectrom.45, 703–714 (2010).
- X.Y. Yang, P. Neta, and S.E. Stein, Anal. Chem.86, 6393–6400 (2014).
- H. Oberacher, The Wiley Registry of Tandem Mass Spectral Data, MSforID, 1st Ed. (John Wiley & Sons, Hoboken, New Jersey, 2011).
- R.L. Fitzgerald, J.D. Rivera, and D.A. Herold, Clin. Chem.45, 1224–1234 (1999).
- K.L. Lynch, A.R. Breaud, H. Vandenberghe, A.H.B. Wu, and W. Clarke, Clin. Chim. Acta411, 1474–1481 (2010).
- R. Mylonas, Y. Mauron, A. Masselot, P.A. Binz, N. Budin, M. Fathi, V. Viette, D.F. Hochstrasser, and F. Lisacek, Anal. Chem. 81, 7604–7610 (2009).
- H. Oberacher, G. Whitley, B. Berger, and W. Weinmann, J. Mass Spectrom. 48, 497–504 (2013).
- H. Oberacher, M. Pavlic, K. Libiseller, B. Schubert, M. Sulyok, R. Schuhmacher, E. Csaszar, and H.C. Kofeler, J. Mass Spectrom.44, 494–502 (2009).
- M. Pavlic, K. Libiseller, and H. Oberacher, Anal. Bioanal. Chem.386, 69–82 (2006).
- M. Pavlic, B. Schubert, K. Libiseller, and H. Oberacher, Forensic Sci. Int.197, 40–47 (2010).
- H. Oberacher, M. Pavlic, K. Libiseller, B. Schubert, M. Sulyok, R. Schuhmacher, E. Csaszar, and H.C. Kofeler, J. Mass Spectrom. 44, 485–493 (2009).
- C. Jimenez, R. Ventura, and J. Segura, J. Chromatogr. B767, 341–351 (2002).
- E. Trullols, I. Ruisanchez, and F.X. Rius, Trends Anal. Chem.23, 137–145 (2004).
- S. Cardenas and M. Valcarcel, Trends Anal. Chem.24, 477–487 (2005).
- M. Schulz and A. Schmoldt, Pharmazie 58, 447–474 (2003).
Florian Pitterl is an analytical chemist who received his PhD at the University of Innsbruck, Austria, in 2010. After spending a year as postdoctoral fellow at the National Hellenic Research Foundation in Athens, Greece, he got a postdoc position at the Institute of Legal Medicine of the Medical University of Innsbruck. Since 2014 he has been lab head of the Forensic Toxicology Laboratory at the Institute of Legal Medicine. Sebastian Köb, Johanna Pitterle, and Julia Steger were masters students in the group of Herbert Oberacher. Herbert Oberacher is an analytical chemist who received his PhD at the University of Innsbruck, Austria, in 2002. After spending a year as a postdoctoral fellow at the University of the Saarland, Germany, he got a position as senior researcher at the Institute of Legal Medicine of the Innsbruck Medical University, Austria. In 2007 he received the “venia docendi” for bioanalysis, and in 2011 he was appointed to Associate Professor. His research focuses on the development of chromatographic, mass spectrometric, and bioinformatic techniques for the analysis of bioorganic molecules with special emphasis on small molecules and nucleic acids. He has (co-)authored more than 90 papers in international scientific journals and received several national and international awards.

Best of the Week: Flow Imaging Microscopy, Electric Vehicles, Air Pollution in India
April 11th 2025Top articles published this week include an interview about flow imaging microscopy, a news story on fiber optics and electric vehicles, and a recap of a study that explored the use of micro-particle-induced X-ray emission (micro-PIXE) spectroscopy to trace urban and indoor air pollution sources in India.