Carotenoids—Their Resonance Raman Spectra and How They Can Be Helpful in Characterizing a Number of Biological Systems
The resonance Raman spectra of carotenoids vary with subtle changes on the functional side groups, making these spectra useful for identifying and characterizing carotenoids.
Carotenoids serve multiple uses in biological systems ranging from visual pigments to antioxidants. Because the resonance Raman spectrum varies with subtle chemical changes on the functional side groups, the spectra of carotenoids have been used for identification and characterization. This installment of “Molecular Spectroscopy Workbench” summarizes the spectroscopy of these materials and why it can be useful when studying them.
Like the hemes and chlorophylls, the carotenoids are highly colored materials. The hemes exhibit resonance Raman (RR) spectra of varying intensities that depend on the excitation wavelength and the lifetime of the resonant electronic state. The chlorophylls are highly fluorescent, sometimes with a resonance Raman spectrum, but the carotenoids always exhibit RR spectra. Whether an unsaturated system produces RR spectra, fluorescence, or both, depends on the lifetimes of the intermediate electronic states that are excited by a laser beam. A study I did of a series of hemeproteins in the 1970s enabled me to document this phenomenon by relating the intensities of the RR signal and real porphyrin fluorescence to the lifetimes of the heme electronic states in this system (1).
Modeling the Spectra of Carotenoids with Polyenes
The carotenoids are a class of highly colored materials whose electronic absorption is derived from the polyene backbone of the molecule. A polyene is a hydrocarbon chain with alternating double and single bonds between the carbon atoms. The π electrons from the double bonds are conjugated onto the single bonds; the lowered electron density results in electronic states of lower energy. Whereas a single ethylenic bond absorbs light in the ultraviolet (UV) part of the spectrum, a polyene absorbs in the visible part of the spectrum, and the longer the polyene chain, the lower the energy of absorption. In addition, the lower bond order between the carbon atoms lowers the vibrational energy of the carbon–carbon double bond; a pure ethylenic bond frequency is close to 1650 cm-1, but the Raman frequency of polyenes is lower than 1600 cm-1, with longer chain polyenes producing lower frequency Raman bands.
The effect of the polyene chain length on the wavelength of the peak in the optical absorption spectrum and the frequency of conjugated double bonds was studied in the 1970s (2) and 1980s (3) by Gerrard and Maddams, who were then working at the British Petroleum Company and characterizing the degradation of poly vinyl chloride (PVC). The thermal degradation of PVC proceeds by the elimination of a proton and chlorine ion across a C-C bond, leaving a >C=C< behind. This acts as an initiation site for additional evolution of HCl, which is sequentially extracted along the chain, “unzipping” and forming conjugated polyene sequences. They found that seven or more conjugated double bonds placed the absorption in the visible part of the spectrum, consequently “discoloring” the product. However, the presence of the electronic absorption of the conjugated chain produced “resonance” conditions for the Raman spectrum. Under resonance conditions, Gerrard and Maddams (3) reported that polyenes with nine or more double bonds could be detected down to 0.00001%! This sensitivity will be important in detecting carotenoids in various biological matrices. In addition, Gerrard and Maddams observed that the frequency of the >C=C< double bond varies with N, the number of carbons in the conjugated chain; as the chain gets longer, the Raman frequency drops because the bond order of the double bond drops. Even earlier it had been observed that the Raman frequency of short conjugated chains varied with the log N (4). But it would be incorrect to extrapolate this relationship to very long chains because the frequency clearly cannot approach 0 cm-1. Gerrard and Maddams used the value of 1474 cm-1 for the infinitely long chain, and they attempted to fit the measured spectra of degraded PVC to expectations based on the range of N values in any sample (3).
A related material known as polyacetylene is described as an infinite polyene chain. The Raman spectrum of this material has also been studied at different excitation wavelengths, and its spectrum is seen to vary with the laser wavelength, which implies that the conjugation length in this material is inhomogenous as well (5).
For both degraded PVC and polyacetylene there have been attempts to develop a rigorous relationship between the chain length and the Raman frequency, but because of the lack of homogenous samples, this relationship has to be modeled using the proposed distribution function for the conjugation chain lengths. At any event, studies of both types of materials confirm that the Raman frequency of the carbon double bond drops with conjugation length. Of additional interest is the behavior of the cis modification, which has been well-characterized for the polyacetylene. Whereas the Raman spectrum of the trans-polyacetylene is dominated by two bands (the carbon–carbon single bond between 1050 and 1150 cm-1, and the carbon–carbon double bond between 1450 and 1550 cm-1), the cis form has three backbone vibrations at 909, 1250, and 1541 cm-1. In addition, in the cis form there is a long progression of overtones and combinations as well as two broad luminescent bands at about 1.9 eV (652 nm) and 1.3 eV (9.54 nm). The luminescent bands always appear at these energies, and the Raman frequencies do not shift with excitation wavelength, although the Raman intensity varies widely and follows approximately the absorption spectrum. One can infer that the cis form is more homogeneous in its conjugation length, and the excited electronic states are long enough lived to exhibit a luminescent decay similar to that seen in some of the hemes (1). Referring again to the first Gerrard and Maddams publication (2) we see that the spectrum of the thermally degraded PVC that they studied exhibits combinations and overtones and a fluorescent background like the cis form of polyacetylene, implying that the unzipping process may produce the cis form of the product.
Carotenoids are natural products with chain lengths of four to more than 15 conjugated units. They cannot be synthesized by animals, so they must be consumed in the diet. Because of the presumed importance in animal nutrition, the occurrence of these materials is of commercial interest. For instance, the highest cost in the farming of salmon, whose color is derived from astaxanthin that is ingested from their diet in the ocean, is the cost for the carotenoids in the feed for the farmed fish.
Carotenoids absorb light with wavelengths between 400 and 550 nm, which produces colors ranging from yellow to red. In plants and algae, they absorb light to pass energy on to photosynthetic centers for energy storage, and they also absorb light to protect chlorophyll from photodamage. Some carotenoids can be converted to retinol for light detection in the eye. Three carotenoids (lutein, astaxanthin, and zeaxanthin) are present in the eye to absorb short-wavelength light and protect the macula in the retina. A short chain called retinal is responsible for vision, but it serves other functions as well; later in this article we enumerate some places where carotenoids are found and discuss some of their functionalities.
Some time ago Professor Gene Hall from Rutgers University visited with a collection of fish oil dietary supplements to explore how their quality could be assessed by Raman measurements. The goal was to detect and quantify unsaturation, and possibly trans double bonds, which are believed to be unhealthy. I was fairly shocked to record the spectrum shown in Figure 1 from a commercial sample of krill oil. Even though the sample was totally colorless, the intensity from the carotenoid was so intense that the sample of the fish oil itself was not visible. (The nonresonant enhanced fish oil spectrum would have had strong bands in the mid 1400 cm-1 region for the >CH2 deformation, 2800–2900 cm-1 for the saturated CH, and ~1650 cm-1 for the >C=C< band.) The label on the bottle did not even indicate that a carotenoid was present! Figure 1 shows the spectrum excited at 532 nm; the spectrum has been baseline subtracted.
Figure 1: Raman spectrum of a commercial sample of krill oil excited at 532 nm and recorded on an Aramis Raman spectrometer (Horiba) with a 1200-g/mm grating. A D1 neutral density filter was used to prevent saturation during the integration.
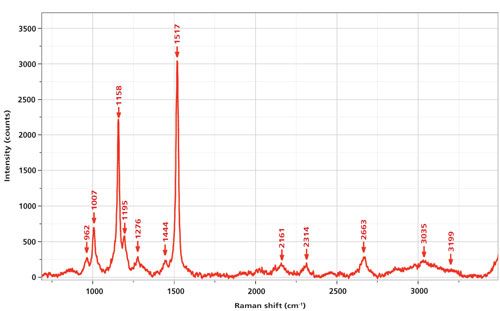
At the time that these measurements were made, we tentatively assigned the spectrum to that of astaxanthin, a modification of β-carotene in which carbonyl and hydroxyl groups have been added symmetrically to the rings. This assignment was based on a review article published in 1985 on the carotenoids (6). Figure 1 of that article shows spectra of β-carotene, astanxanthin, and β-apo-8′-carotenal. Although the spectrum of astaxanthin is the best match to the spectrum in our Figure 1 here, some of the band frequencies match well while others do not; in particular the >C=C< stretch in the Merlin publication (6) appears at 1523 cm-1, significantly different from what we see in our spectrum at 1517 cm-1. However, a more contemporary publication that discusses the various isomers of astaxanthin provides an almost precise match to the all-trans form (7). In addition, this publication indicates that a frequency of 1523 cm-1, as seen in the Merlin publication, is close to that of one of the cis isomers isolated by chromatography.
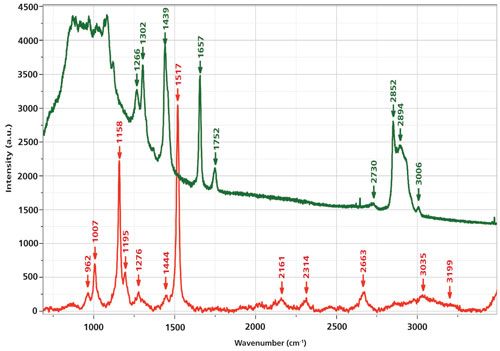
In my Figure 1 I have also labeled bands between 2000 and 3500 cm-1. Simple arithmetic shows that these frequencies fit overtones and combinations of the fundamentals between 750 and 1750 cm-1. To further support the assignment of this spectrum to that of a carotenoid, rather than the oil in which it is dissolved, Figure 2 shows the spectrum of the carotenoid overlaid with that of a commercial olive oil. The strongest bands in the spectrum of the olive oil are 1302 cm-1, the backbone twist; 1439 cm-1, the >CH2 deformation; 1657 cm-1, the olefinic bond (>C=C<); 1752 cm-1, the >C=O from esterification with the glycerol; 2852 and 2894 cm-1, the saturated CH stretches; and 3006 cm-1, the olefinic CH stretch. Although olive oil is a triglyceride of mostly the ω9 fatty acid (oleic acid, with one double bond), which is a bit different from fish oil, for my purposes here it can be used for comparison. Fish oils are composed of triglycerides of ω3 fatty acids with four or more double bonds. The double-bond frequency may shift a bit, but its intensity relative to everything else in the spectrum will be enhanced because of the higher content of the >C=C< bonds. However, the point is that the spectrum that we measured of the carotenoid has the characteristics of resonance Raman conditions and could never be confused with that of an edible oil.
Figure 2: Raman spectra of krill oil, which shows only the spectrum of a carotenoid (bottom) versus the spectrum of olive oil in which bands of the backbone, CH2 deformation, >C=C<, >C=O, and CH have been labeled (see text).
Understanding the Raman Spectra of Carotenoids in Terms of Model Polyenes
Monitoring the Presence of Carotenoids in Natural Products
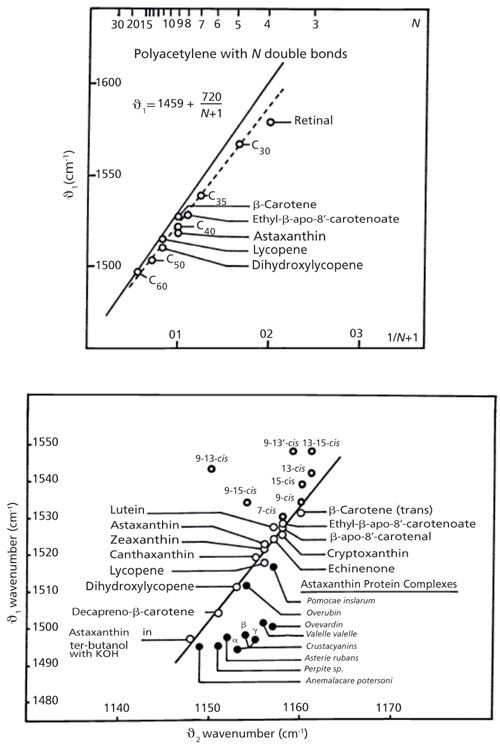
There are currently a fair number of studies of carotenoids for a variety of applications. But it is interesting to examine further Merlin’s 1985 review (6). In Figure 3 (top) I have reproduced his plot of the >C=C< frequency as a function of 1/(N+1), where N is the number of double bonds in polyacetylene and in a series of carotenoids. Although the carotenoid points do not fall precisely on the polyacetylene line, the trend is remarkably similar. In the bottom of Figure 3 I reproduced his plot in which, for each carotenoid, there is a point representing the C-C single bond frequency on the x-axis, and the C=C double bond frequency on the y-axis. Many of the points hug a line. However, there is a cluster of points on the upper left representing the cis isomers and another interesting cluster of protein complexes on the lower right. In the case of the proteins, the presumption is that the protein environment affects the conformation or bond order of the carotenoid. In the case of the cis isomers, the kink in the chain affects the extended π system, with the effect being greatest when closest to the center of the chain. Merlin also cites earlier work showing the effect of the various cis isomers on the spectrum in the single bond region. At the conclusion of that article he cites a variety of in situ measurements indicating a knowledge of the potential of Raman analysis of biological materials with carotenoids, with a comment that the field will expand as the instrumentation develops. Reading this today one is struck by the insights that were already available 30 years ago!
Figure 3: Top: The relationship between the >C=C< double bond and the number of conjugated units for polyacetylene and for some carotenoids. Bottom: The interdependence between the single bond frequency and double bond frequency in a number of carotenoids. Adapted with permission from reference 6.
The physical understanding of polyconjugated molecules has advanced recently with the development of the effective conjugation coordinate theory (ECC) (8). This new theory was applied to determine the structure of the pigment in Corallium rubrum, a coral containing a polyconjugated pigment that cannot be purified (9). In extending the ECC theory to carotenoids and psyttacofulvins (analogues of the carotenoids without the pendant methyl groups on the polyene chain), they noted that the presence of the methyl groups will distort the planar geometry of a bare polyene. This distortion removes the center of symmetry, which has the consequence of producing a band near 1050 cm-1, which is the strong infrared (IR)-active mode assigned to the in-plane rocking of the -CH3 groups. In addition, they noted the significant effect of the presence of methyl groups on the frequency of the C-C bonds. They argued that the presence of a methyl group presents a mechanical disruption of motion on the chain; as the number of uninterrupted C-C bonds increases, the frequency of the collective motion decreases.
In summary, the resonance-enhanced Raman spectrum of carotenoids is sensitive to
- the number of conjugated units,
- the presence of methyl groups, and
- molecular shape such as twisting and trans versus cis conformation.
Exploiting the Raman Spectra of Carotenoids
Because of the biological and economical importance of carotenoids, several authors have made in situ measurements in plant and algal material, and compared the results to measurements of extracted material (10,11). Both of these groups worked with a Fourier transform (FT) Raman system to take advantage of the total lack of matrix fluorescence. Their study of a large number of carotenoids in a large number of plant materials confirmed the following:
- the dependence of the double bond frequency on the number of conjugated bonds,
- that modifications to the terminal groups of the chains have subtle effects on the observed spectra,
- that there are matrix effects on the spectra-that is, the spectra are affected by environmental interactions,
- that the spectra of extracted material in solvents are different from those in the plants, and
- that isomerization can be monitored.
Huang and colleagues (12) examined the spectra of individual algal cells of two species and determined that in healthy cells only carotenoids were detected, whereas in nitrogen-deprived cells, the measured spectrum represented a combination of carotenoid, triglycerides, and chlorophyll. In addition, they performed microscopic mapping that showed clearly widespread distribution of carotenoid in healthy cells, and a distinct lipid droplet (without carotenoids) in the nitrogen-deprived cell.
Withnall and colleagues (13) also studied carotenoids in natural products, but in their case they examined sea shells in addition to plant material. Their plot of the double-bond frequency as a function of 1/(N + 1) spans the range between about 1580 cm-1 for retinal (four double bonds) to about 1490 cm-1 for dodecapreno-β-carotene (17 double bonds). I remember some years ago we had a visitor who was measuring carotenoids in mollusk embryos because each species has a different carotenoid. In the microscope view the embryos looked exactly like the adult mollusks.
Bioclinical Studies: Some Examples
Ramanauskaite and colleagues (14) measured carotenoids in lymphocytes collected from peripheral blood of healthy people. Raman images showed that the carotenoids collected in the Gall bodies. They found that the level of carotenoids dropped with age. Because it is known that carotenoids have immunomodulating activity at physiological concentrations, there is a possibility that this could be the origin of their known anticancer effect. In addition, because prospective and retrospective epidemiological studies show that dietary carotenoid is negatively correlated with some cancers, they measured the carotenoid levels in lymphocytes of lung cancer patients and found that these patients had reduced levels of carotenoids in their Gall bodies, compared to those of healthy patients.
Kutuzov and colleagues (15) published a study of orientation of carotenoids in myelin membranes with the goal of determining whether the carotenoids play a role in facilitating or regulating electrical conduction by the myelin. This impressive study takes into account sample optical effects and rigorous orientation–polarization analysis. The results produced orientation distribution functions (ODFs) for both carotenoids and lipids. The ODF shows that there is preferential orientation of the carotenoids in the radial direction in the nerve fiber.
In an attempt to assess the carotenoid status of individuals, RR spectra (473-nm excitation) were recorded through the palm skin of 81 participants. A “Raman score” was plotted relative to the following parameters for each individual: carotenoid sum, β-carotene, lycopene, lutein-zeaxanthin (these were determined from serum), and fruit and vegetable intake scores for season 1 and season 2, which would account for the availability of different fruits and vegetables in the summer versus the winter. The authors state “this study show(s) a strong and significant positive correlation between the field RRS score and serum carotenoids.” To my eye, the plots show an inordinate amount of scatter, but maybe for such an application this is acceptable.
Bernstein and colleagues (16) measured carotenoid levels in the macula of normal subjects and those with age-related macular degeneration (AMD) using a 488-nm laser for Raman excitation. They found that the carotenoid level decreased with age, even in normal subjects, but in AMD patients not taking lutein supplements the levels of lutein and zeaxanthin were 32% lower than normal elderly controls. Patients who had initiated high doses of supplements had levels in the normal range. It was suggested that RR measurements of macular pigments could facilitate a prospective clinical study of lutein and zeaxanthin protection.
The Visual Process
The human visual pigment, rhodopsin, in its resting form, is a Schiff-base complex of 11-cis-retinal, the aldehyde of half of a β-carotene molecule, which is bound to the transmembrane G-protein receptor, opsin, via the terminal amine group on a lysine amino acid. In the visual process, a photon causes isomerization of 11-cis-retinal to the all-trans form, which subsequently requires rearrangement of the protein and that, in turn, initiates the electrical signal that is sent to the brain and sensed as light (17). George Wald received the Nobel Prize in 1967 for his investigation of the various intermediates in this process (18). For the readers of this column installment, it is important to recognize that the optical absorption spectra of the various forms of rhodopsin exhibit shifting peaks in their absorption spectra because changes in the electron distribution in the various isomers of the polyene chain will sensitively affect the electronic states that are probed by optical absorption. Consequently, the resonance Raman signal will also reflect the changing conditions of the molecule. In 2001 Kandori, Shichida, and Yoshizawa (a collaborator of Wald) reviewed what was known of the photoisomerization in rhodopsin (19). By then, pump–probe measurements with femtosecond resolution had enabled documentation of the details of the isomerization cascade, capturing excited state dynamics. They note that the photoreaction of the chromophore in the protein is faster than in solution. RR spectra of samples trapped at low temperature were compared to pump–probe measurements to support the conclusions being made-that at low temperatures isomerization cannot proceed. In the same year the group of Richard Mathies reported “picosecond time-resolved resonance Raman spectroscopy . . . to probe the structural changes of rhodopsin’s retinal chromophore as the cis-to-trans isomerization reaction . . . initiates vision” (20). They showed that the chromophore is in an all-trans, strained conformation within 0.8 ps. There is presumably an ultrafast localized protein response, but additional delocalized protein changes need longer times and therefore large amounts of energy are stored for subsequent protein conformational changes. Thus, understanding the biophysics of the light interacting with the rhodopsin, the biochemistry of the structure of the complex, and how it changes with time, provides an understanding of the physiology of the visual process.
Summary
My goal in this column was to provide some insight into the usefulness of the Raman spectra of carotenoids. There are many of them-Wikipedia states that there are more than 600 known, some that contain oxygen, and others that do not. Because of the extended π electron system they absorb light in the visible part of the electromagnetic spectrum which in turn facilitates a Raman spectrum that is strongly enhanced. I started by describing the origin of the electronic properties by citing a description of polyene from the physics literature. I cited some studies in the field of analytical polymer chemistry where workers determined that the color that appears in thermally degraded polyvinyl chloride is a result of the evolution of HCl from the polymer and the polymer’s further unzipping into a polyene. Then I discussed, in a general way, the spectra of the carotenoids. Finally, I cited a few examples of places where these spectra have been used to extract biochemical information. As always, this is not an exhaustive review. I am absolutely certain that my citations do not include everything important that has been done. My goal was to enable someone who might be interested in exploring the RR spectra of carotenoids to understand enough to find what they need in the literature.
References
- F. Adar, M. Gouterman, and S. Aronowitz, J. Phys. Chem.80, 2184–2191 (1976).
- D.L. Gerrard and W.F. Maddams, Macromolecules8, 54 (1975).
- D.L. Gerrard and W.F. Maddams, Macromolecules14, 1356–1362 (1981).
- P.P. Shorygin and T.M. Ivanova, Sov. Phys. - Dokl. (Engl. Transl.) 8, 493 (1963).
- D.B. Fitchen, Mol. Cryst. and Liquid Cryst. 83, 95–103 (1982).
- J.C. Merlin, Pure & Appl. Chem. 57(5), 785–792 (1985).
- B. Subramanian, N. Tchoukanova, Y. Djoued, C. Pelletier, M. Ferron, and J. Robichaud, J. Raman Spectrosc. 45, 299–304 (2014).
- G. Zerbi, in Vibrational Spectroscopy of Polymers: Principles and Practice, N.J. Everall, J.M. Chalmers, and P.R. Griffiths, Eds. (John Wiley, Hoboken, New Jersey, 2007), pp. 488–536.
- L. Brambilla, M. Tommasini, G. Zerbi, and R. Stradi, J. Raman Spectrosc. 43, 1449–1458 (2012).
- H. Schulz, M. Baranska, and R. Baranski, Biopolymers77, 212–221 (2005).
- V.E. deOliveira, H.V. Castro, H.G.M. Edwards, L. Fernanco, and C. de Oliveira, J. Raman Spectroscopy41, 642–650 (2010).
- Y.Y. Hunag, C.M. Beal, W.W. Cai, R.S. Ruoff, and E.M. Terentjev, Biotechnology and Bioengineering DOI 10.1002/bit.22617 (2009).
- R. Withnall, B.Z. Chowdhry, J. Silver, H.G.M. Edwards, and L.F.C.de Oliveira, Spectrochim Acta A59, 2207–2212 (2003).
- R.B. Ramanauskaite, I.G.M.J. Segers-Nolten, K.J. deGrauw, N.M. Sijtsema, L. van der Maas, J. Greve, C. Otto, and C.G. Figdor, Pure & Appl. Chem.69(10), 2131–2134 (1997).
- N.P. Kutuzov, A.R. Brazhe, G.V. Maksimov, O.E. Dracheva, V.L. Lyaskovskiy, F.V. Bulygin, and A.B. Rubin, Biophys. J.107, 891–900 (2014).
- P.S. Bernstein, DY Zhao, S.W. Wintch, I.V. Ermakov, R.W. McClane, and W. Gellermann, Ophthalmology109(10), 1780–1787 (2002).
- “The Molecular Basis of Visual Excitation,” G. Wald, Nobel Lecture, 1967.
- H. Kandori, Y. Shichida, and T. Yoshizawa, Biochemistry (Moscow) 66(11), 1197–1209 (2001).
- J.E. Kim, D.W. McCamant, L. Zhu, and R.A. Mathies, J. Phys. Chem. B105(6), 1240–1249 (2001).

Fran Adar is the Principal Raman Applications Scientist for Horiba Scientific in Edison, New Jersey. Direct correspondence to: SpectroscopyEdit@UBM.com

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.