Combining Thermal Desorption GC and TOF-MS for the Determination of Melon VOC Profiles
Special Issues
The quality and safety of ready-to-eat packaged foods-such as salads-is very difficult for consumers and suppliers to judge, and improving this situation is the focus of a Europe-wide research project. Part of the project is devoted to the development of better methods to detect and analyze the volatile organic compounds released from relevant food types, in an effort to identify biomarkers for quality and microbial contamination. This article examines one important food (melon) and shows how a method based on thermal desorption (TD) with gas chromatography-time-of-flight-mass spectrometry (GC–TOF-MS) can elucidate how key volatiles vary with time of storage and with the size of the melon pieces. The article highlights how such analytical information will be of value in efforts to improve the quality and safety of ready-to-eat foods.
The quality and safety of ready-to-eat packaged foods-such as salads-is very difficult for consumers and suppliers to judge, and improving this situation is the focus of a Europe-wide research project. Part of the project is devoted to the development of better methods to detect and analyze the volatile organic compounds released from relevant food types, in an effort to identify biomarkers for quality and microbial contamination. This article examines one important food-melon-and shows how a method based on thermal desorption with gas chromatography-time-of-flight mass spectrometry (GC–TOF-MS) can elucidate how key volatiles vary with the size of the melon pieces. The article highlights how such analytical information will be of value in efforts to improve the quality and safety of ready-to-eat foods.
Currently there are few objective criteria for determining the safety and quality of fruit and vegetables. People working in the industry estimate shelf life mainly by judging the appearance of the product. This approach is limited because although appearance is related to overall quality, it does not necessarily indicate deficiencies in the aroma profile or other important characteristics such as nutrient content or the presence of pathogenic microorganisms.
The issue is particularly acute for fresh produce that undergoes processing before consumption. With the produce usually cut into pieces for convenience of the consumer and no sterilization step to eliminate microbes, microbial deterioration can progress unhindered and any contamination with pathogenic bacteria could potentially reach dangerous levels. This possibility is concerning given the rise in popularity of these foods-especially in light of governmental initiatives to encourage consumption of healthier foods.
A project funded by the European Commission through the 7th Framework Program aimed to improve this situation. The initiative, known as QUAFETY (1) (a combination of the words quality and safety), aimed to improve the quality and safety of ready-to-eat fresh produce by working with the industry to develop new diagnostic tools for the evaluation of quality, in terms of both flavor and nutrients, for the determination of shelf life, and for the detection of microbial contamination. It is anticipated that the tools developed by QUAFETY will aid industry operators with their decision-making at critical points in the fresh-cut food supply chain, ultimately increasing quality and nutritional value while minimizing waste and food safety risks to consumers.
With this aim in mind, the QUAFETY initiative explored rapid, nondestructive analytical methods that can quantify and manage spoilage and pathogenic microorganisms. A project that has been developing markers for nutritional and functional quality is also contributing to this process. One aspect of this project is the evaluation of various methods for collection of volatile organic compounds (VOCs) and investigating how these data can be correlated to other measures of quality developed by the wider QUAFETY team.
The foods under investigation were arugula (rocket), strawberry, and melon-representative of freshly prepared salads and fruit salads, respectively.
The Aroma Characteristics of Melon
With their characteristic aroma, melons (Cucumis melo) are an important component of fresh fruit salads. This aroma is particularly associated with cultivars of the group
Cantaloupensis-the cantaloupe melons-that produce esters, sulfur-containing aromatics, short-chain alcohols and aldehydes, sesquiterpenes, and norisoprenes (2). Cantaloupe melons were a focus of our studies because of their well-known aromaticity, but also because they have a limited shelf life (2), and so they are highly relevant to investigating quality issues surrounding prepackaged fruit salads.
Various factors are known to affect the VOC profiles of melon. Unsurprisingly, the diversity and abundance of VOCs rises with increasing maturity of the harvested melon (3), but it also undergoes changes during storage (4)-changes that can be minimized by storing the melon at 4 °C (5,6). The initial processing of the melon also plays a key role in the VOC profiles and the perceived quality (7,8), but the size of the pieces used in fruit salads has received little attention. This is partly because the majority of previous research was performed using homogenized material (3,4,8,9).
Solid-phase microextraction (SPME) is widely used in the food industry as a means of extracting volatile compounds from headspace. However, although the technique is very easy to implement, analytes are difficult to quantify. Automation also presents significant challenges when working with perishable samples such as melon. Previous authors (3) have also pointed out the difficulty of using SPME fibers as the extraction medium for melon, because of the lack of reproducibility over time, with analyte type, and between media containing different concentrations of nonvolatile chemicals.
The use of thermal desorption (10) avoids such problems, and because the metal sorbent tubes used are robust and secured with diffusion-locking inserts, samples can be collected and then transported to the laboratory without risk of sample loss or contamination. An additional benefit is that desorbed samples can be split, and the unanalyzed part of the sample recollected onto the same sorbent tube (or a second, clean one)-either as a back-up or to be analyzed again with, for example, a different split ratio, allowing a very wide concentration range to be easily handled (11).
Expanding on a previous report (12), this study describes how thermal desorption with gas chromatography-time-of-flight mass spectrometry (GC–TOF MS) can be used to investigate the VOC profiles of processed melon, and how these volatile profiles vary with the size of the cut pieces. A linear discriminants analysis was used to identify the VOC components that were best able to discriminate between melon pieces of different sizes.
ExperimentalSample Preparation
Commercial cantaloupe melons (Cucumis melo L. “Arapaho”) were harvested and processed as previously described (12).
Briefly, melons of uniform appearance were cooled overnight to 5 °C, washed in cold water, dipped in sodium hypochlorite solution (100 μg/L) for 2 min, rinsed with deionized water, and allowed to drain. The skin, blossom and stem ends, placental tissue, and seeds were removed. Using a sharp knife, melons were cut into three shapes and sizes: cubes with a 10-mm edge (small), trapezoids with a 15-mm edge (medium), and trapezoids with a 25-mm edge (large). Within each of these three cut sizes, individual pieces prepared from numerous fruit were randomized, and approximately 150 g was placed in polypropylene trays and stored at 4 °C. Samples were taken for analysis after 1, 4, 7, 10, and 15 days.
VOC Sampling
As previously described (12), at each time point the tray was inserted into a multipurpose roasting bag (25 cm × 38 cm, TJM). The bag was sealed and stored at 20 °C for 1 h before sampling 200 mL of headspace onto thermal desorption tubes fitted with SafeLok diffusion-locking inserts and packed with Tenax TA and SulfiCarb (Markes International), using an Easy-VOC grab-sampler (Markes International). Control samples were collected from empty bags and retention standards were prepared by loading 1 μL of a C8-C20 alkane standard directly onto a thermal desorption tube.
Thermal Desorption-Gas Chromatography
Tubes were desorbed using a TD-100 thermal desorption system (Markes International) as previously described (12).
Tube desorption: 10 min at 280 °C, trap flow of 40 mL/min, “Sulfur” cold trap (Markes International), trap low 25 °C. Trap desorption and transfer: 40 °C/s to 300 °C (3 min), split flow of 20 mL/min into a GC system (7890A, Agilent Technologies). Column: 60 m × 0.32 mm, 0.5-μm df Rxi-5ms (Restek). Continuous flow: 2 mL helium. Temperature program: 35 °C (5 min), then 5 °C/min to 100 °C, then 15 °C/min to 250 °C (5 min).
Mass Spectrometry
As described previously (12), mass spectra were recorded over the m/z 30–350 range on a time-of-flight mass spectrometer (BenchTOF-HD, Markes International), following dynamic baseline compensation to remove unwanted minor background components. For the statistical analysis, data was deconvoluted and integrated using AMDIS (NIST 2011) with a custom retention-indexed mass spectral library based on the NIST 11 database. Compounds that were not abundant in all replicates of at least one single cut size were excluded from statistical analyses, as were compounds abundant in controls. Areas of remaining compounds were normalized within samples and standardized for each compound.
Statistical Analysis
Differences between headspace profiles were assessed with PerMANOVA (function Adonis in vegan package) and canonical analysis of principle coordinates (function capscale in biodiversity package). The number of compounds was reduced using principal components analysis (PCA) and loadings of the first six principal coordinates, and retesting of differences with the reduced number of compounds.
Results and Discussion
Selection of Sampling Methodology
Initial studies focused on development of an optimum method for sampling and analyzing the melon headspace.
To ensure that sample collection was as simple as possible, a manually operated grab-type sampler was used, with capture of melon headspace directly on to a sorbent-packed tube. Best results were found using thermal desorption sorbent tubes packed with Tenax TA (a sorbent that is able to trap a wide volatility range) with SulfiCarb (which is active for very volatile compounds including reactive sulfur species). The choice of desorption temperatures ensured good chromatographic performance across the volatility range.
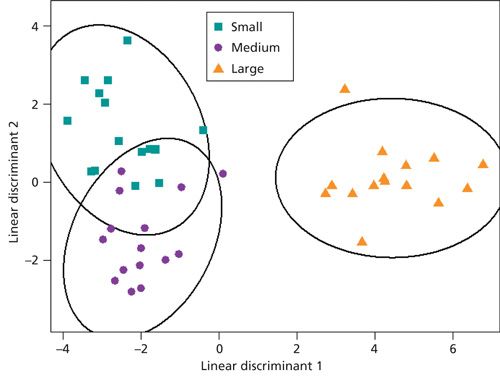
Figure 1 shows an example chromatogram from the small cut size melon, and Figure 2 shows an expansion of a region containing a number of minor components.
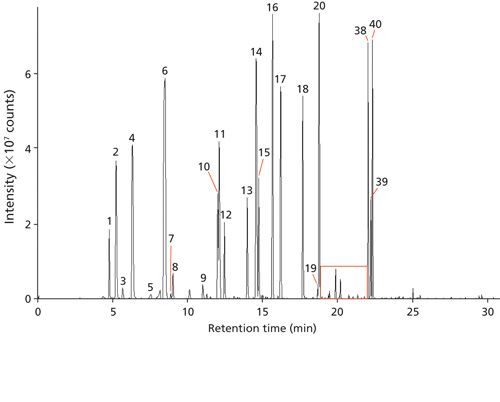
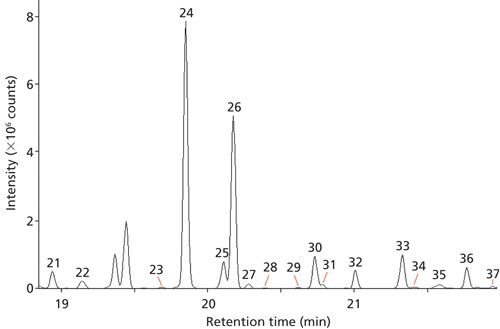
Key Volatiles and Spectral Matching
The time-of-flight mass spectrometer used in this study provided the high sensitivity that is expected from such instruments, boosted in this case by a direct-extraction technique that reduced ion wastage. The inherently better sensitivity of TOF instruments, however, normally has to be weighed against their inability to generate mass spectra that match with those in commercial (usually quadrupole-acquired) databases. Typically, the solution would be to generate a custom TOF MS spectral library, at considerable expense of time.
This inconvenience was circumvented here by the ability of the TOF instrument to acquire spectra that do match with those from quadrupoles. This is demonstrated to good effect in Figure 3, which shows how the instrument was able to confidently distinguish two isomeric esters (peak 9 and peak 12) and from the melon headspace, despite their rather similar spectra (Figure 3). Note, in particular, the high-quality matching of the molecular ion cluster for methyl butanoate, despite its low abundance.
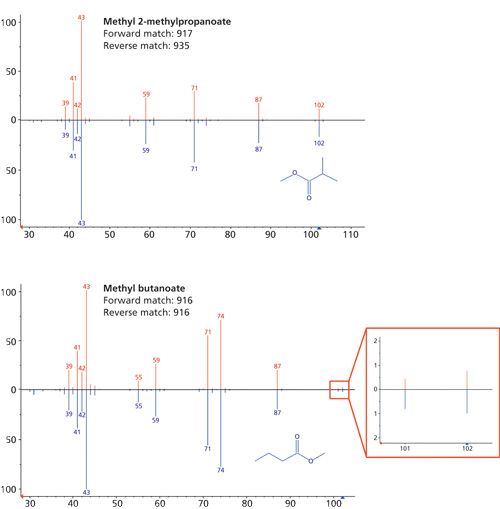
Such capability was useful here to confirm tentative identifications made on the basis of retention indexing. Indeed, the analysis showed that, as expected, esters predominated among the volatiles, including a number known (9) to contribute to the distinctive aroma of melons: ethyl 2-methylpropanoate (peak 13), methyl 2-methylbutanoate (peak 15), ethyl butanoate (peak 16), ethyl 2-methylbutanoate (peak 18), 3-methylbutyl acetate (peak 19), 2-methylbutyl acetate (peak 20), ethyl hexanoate (peak 38), and hexyl acetate (peak 40).
Sulfur compounds generally are important in the aroma profiles of a large range of foods, and in the case of melon, ethyl (methylthio)acetate (peak 36, Figure 4) is of particular interest (9).
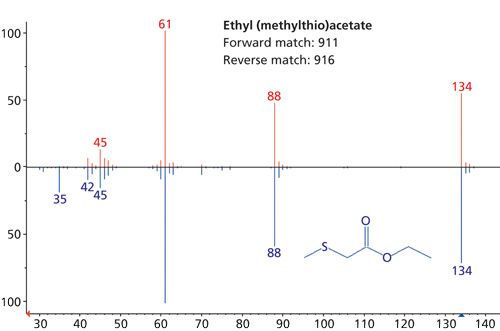
This compound was again identified confidently despite its relatively low abundance in the overall aroma profile.
It is worth noting that the added sensitivity of TOF instruments also offers a particular advantage for samples that do not have an extensive literature on their volatile content. With sensitivity approximating that of selected ion monitoring mode on a quadrupole MS system, but with the ability to acquire spectra across the full mass range, TOF instruments such as that used here make it much easier to screen the entire sample composition.
Statistical Analysis
Data relating to the three cut sizes previously collected (12) were reanalyzed to assess if improved discrimination could be retained when only a restricted number of VOCs was considered. This would be an advantage, because it would enable the identification of potential markers for cut size. The statistical approach used an ordination plot with linear discriminants from a constrained ordination analysis (canonical analysis of principal ordinates). The method compares the full complement of compounds in each headspace profile using different cut sizes as the constraint. Importantly, the VOCs in the headspace profile are treated as dependent variables (13)-unlike PCA, which treats all compounds as independent variables and in which constraints are applied after reduction of variables, usually by application of discriminant function analysis. In essence, this means that the analysis directly compares headspace profiles at different times and from different sizes before reducing its complexity using other methods such as PCA.
Based on a restricted number of VOCs, the small and medium cut sizes were clearly discriminated from the large cut size, although the small and medium cut sizes displayed some overlap (Figure 5). A subset of 21 compounds (mostly esters) from the full list of identified compounds preserved much of the discrimination observed in PerMANOVA (P<0.001). The variation shown within categories indicates that even small differences in wounding responses between and within cut sizes were detected-which in turn may be a result of the high sensitivity of the thermal desorption GC–MS method used.
Conclusions
We have shown here how the combination of grab-sampling with thermal desorption and GC–TOF-MS is a valuable technique for assessing the headspace profiles of melon, and investigating the effect of cut size on the VOCs present.
A notable advantage of this approach is sensitivity. In particular, the combined sensitivity of thermal desorption and TOF-MS ensures that the ability to identify key trace-level components is maximized, while the generation of National Institute of Standards and Technology (NIST)-quality spectra eliminates any uncertainty over component identity.
As with any study of aroma-active components, dynamic range is important. The TOF-MS instrument and the thermal desorption technique again work together to ensure that both high- and low-level components are accurately quantitated.
Additionally, the sampling technique brings practical benefits for a multinational project of this nature. The robustness of the sampling tubes allows samples to be acquired by multiple partners in a global project and transported to a single central laboratory for analysis on the same instrument. The stability of components on the sample tubes is crucial to achieving this, with the sorbents used here being well-validated for storage of a variety of compound types, including sulfur species.
In conclusion, the fact that cut size does affect the VOC profile quite strongly indicates that this factor is important, and should be further investigated. The ability to distinguish between the three cut sizes studied here is encouraging, and shows that this type of analysis can provide a useful benchmark for future studies looking to develop criteria for rapid assessment of the safety of fresh-cut fruit. The ultimate result of this study-through the QUAFETY project-will be the development of unique tools and processes to help the industry make informed decisions about the safety and quality of their products, with additional implications for improving shelf life and reducing food waste.
Acknowledgments
Work was funded by FP7/KBBE2011.2.4-401/289719 - QUAFETY-“Comprehensive approach to enhance quality and safety of ready-to-eat fresh products,” under Work Package 2 (“Process control”). The assistance of Matthew Bates (Markes International) in the development of the project, and Hannah Calder, Laura McGregor, and Caroline Widdowson (all Markes International) in the preparation of this draft, is gratefully acknowledged.
References
(1) QUAFETY, available at: http://www.quafety.eu/.
(2) N.K. Sinha, J.S. Sidhu, J. Barta, J.S.B. Wu, and M.P. Cano, Handbook of Fruits and Fruit Processing, 2nd Edition (John Wiley & Sons, Hoboken, New Jersey, 2012).
(3) J.C. Beaulieu and C.C. Grimm, J. Agric. Food Chem.49, 1345–1352 (2001).
(4) J.C. Beaulieu, HortScience41, 65–73 (2006).
(5) E. Aguayo, V.H. Escalona, and F.A. Rtés, J. Food Sci.69, SNQ148–SNQ155 (2004).
(6) O. Lamikanra, J.C. Chen, D. Banks, and P.A Hunter, J. Agric. Food Chem.48, 5955–5961 (2000).
(7) S.I. Portela and M.I. Cantwell, J. Food Sci.66, 1265–1270 (2001).
(8) J.C. Beaulieu and V.A. Lancaster, J. Agric. Food Chem.55, 9503–9513 (2007).
(9) J.C. Beaulieu, Journal of the American Horticultural Society 127–139 (2006).
(10) E. Woolfenden in Gas Chromatography, C.F. Poole, Ed. (Elsevier, 2012), Chapter 10, pp. 235–289.
(11) Markes International Application Note 101, “Rapid aroma profiling of cheese using a Micro-Chamber/Thermal Extractor with TD–GC-TOF MS,” April 2012.
(12) N.D. Spadafora, I. Machado, C.T. Müller, M. Pintado, M. Bates, and H.J. Rogers, Acta Hortic.1071, 787–793 (2015).
(13) M.J. Anderson and T.J. Willis, Ecology84, 511–525 (2003).

Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.