MS Detection of Waste in Public Swimming Facilities
Special Issues
There is often insufficient prevention to ensure safe swimming environments. Recreation water illness (RWI), most commonly in the form of digestional track illness as well as skin, ear, and respiratory infections, are often caused by water contamination from human waste. Stercobilin is a very stable and suitable chemical biomarker of human waste that has the potential to be used for waste monitoring in public swimming facilities. Using solid phase extraction (SPE) techniques paired with high-resolution mass spectrometry (HRMS), we have developed a robust method used for swimming pool water monitoring to create safer swimming environments.
There is often insufficient prevention to ensure safe swimming environments. Recreational water illness (RWI), most commonly in the form of digestive tract illness as well as skin, ear, and respiratory infections, is often caused by water contamination from human waste. Stercobilin is a very stable and suitable chemical biomarker of human waste that has the potential to be used for waste monitoring in public swimming facilities. Using solid-phase extraction techniques paired with high-resolution mass spectrometry, we have developed a robust method used for monitoring swimming pool water to create safer swimming environments.
Recently, discussions have appeared in mass media publications concerning the adequacy of disinfecting and monitoring harmful bacteria caused from human waste contamination in public swimming facilities (1–4). Much of this discussion stems from a recent study performed by the Center for Disease Control (CDC) in 2012 and published in 2013 (5). In their study, the CDC worked with metro-Atlanta, Georgia, pools to collect samples throughout the active patron months of June–August of 2012. During this study, 1 L of filter backwash samples were collected. Collecting filter backwash is a process in which the flow of water through the filter is reversed so as to remove particulates that have accumulated onto the filter. Samples were then studied using quantitative polymerase chain reaction (qPCR) assays for detection of Pseudomonas aeruginosa and Escherichia coli. Bacterial contamination such as E. coli in public swimming facilities is caused by fecal contamination. The CDC study found that 59% of the monitored pools contained P. aeruginosa and 58% contained E. coli; however, the study did not disclose the actual levels of these bacteria and whether they were harmful (5).
Fecal contaminations can occur in a multitude of ways, such as residual human waste not being removed by prebathing before swimming. Also, small children should either be given bathroom breaks or diaper changes every 60 min. Finally, swimming should be avoided when under gastrointestinal distress. When these bacterial contaminations are not regulated and not under control, exposed swimmers can develop what is known as recreational water illness (RWI) (6). RWI symptoms include gastrointestinal infections (most commonly diarrhea), eye and ear infections, skin irritations, and respiratory infections.
Public pool swimming facility monitoring varies from state to state. In New York state, swimming facilities must maintain a pH between 7.2–7.8 and chlorination must be maintained at a level between 1–3 mg/L for adequate bacterial disinfection. In addition, pool filtration systems must be run at a rate that ensures the entire pool volume is turned over in a 6-h period, and periodic filter backwashing must occur to prevent particulates from reentering the pool (7). Some states, such as Pennsylvania, require additional weekly bacterial testing of public swimming facilities for fecal bacteria (8). States with these rigorous additional bacterial tests face a potential issue of reporting false positives, causing swimming pools to undergo loss of patrons during the time they are closed to address the issue because of delays caused by sampling, handling, and transport in addition to the time the water is analyzed. There is also an issue with the long turnaround time for bacterial test results in scenarios where pools are legitimately undergoing a high bacterial infestation, during which patrons can be exposed and are at risk for RWIs. With this in mind, we are proposing a new method to test for fecal contamination using the human waste metabolite stercobilin.
Previous research has been done using stercobilin and urobilin, a similarly structured human waste compound compared to stercobilin, as potential indicators for fecal waste in natural waters (9–12). These methods have ranged from the use of solid-phase extraction (SPE), high performance liquid chromatography (HPLC), HPLC coupled to mass spectrometry (MS), and fluorescence emissions cavity enhanced spectroscopy. Lower detection limits for these methods have ranged from 0.01 ppb to 0.015 ppb (10,11). Aside from these various methods, capabilities to detect stercobilin from natural waters was demonstrated in a study performed by Miyabara and colleagues that demonstrates a positive correlation in E. coli to stercobilin in water (11). The other important correlation is that during incubation after the water is collected the E. coli will begin to multiply in number, whereas the stercobilin or urobilin concentration remains constant. This trait of stercobilin remaining at a constant concentration during sample transport, with no potential to multiply, can alleviate potential false positive reporting of waste in swimming facilities. Both of these findings presented suggest that stercobilin would make a good indicator for fecal content within public swimming facilities as it directly correlates to the presence of feces bacterial content, and will reduce the number of false-positive feces contamination tests.
ExperimentalMaterials
The following materials were used: stercobilin HCl (Frontier Scientific), Waters Oasis MAX 1 cc SPE cartridges (Waters), isotopically labeled water (18O, 97%, Cambridge Isotope Laboratories, Inc.), methanol (HPLC grade, ≥99.9%, Sigma-Aldrich), acetonitrile (HPLC grade, ≥99.9%, Sigma-Aldrich), ammonium hydroxide (J.T. Baker), trifluoroacetic acid (Fisher Scientific), formic acid 88% (Fisher Scientific), and water filtered using a Thermo Barnstead Nanodiamond filtration system.
Instrumentation
All samples were analyzed using a Hewlett-Packard 1050 series HPLC system coupled to a 12T Bruker Solarix Fourier transform-ion cyclotron resonance (FT-ICR) mass spectrometer. Samples were injected via flow injection using a 20-µL sample loop. The mobile phase consisted of 20:80 (v/v) acetonitrile–water with 0.1% (v/v) formic acid, and the flow rate was 100 μL/min.
Initial SPE Analysis
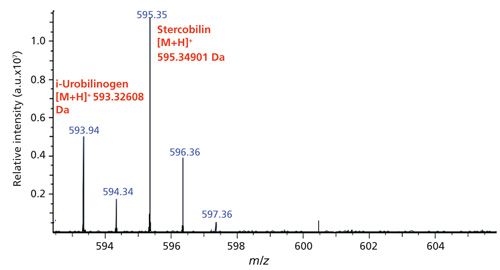
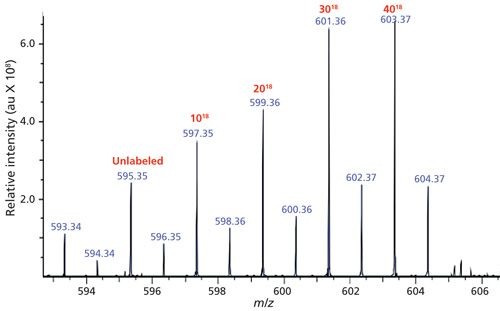
A surrogate standard was prepared in house using a stock solution of stercobilin and isotopically exchanging the carboxylic acid oxygens from 16O to 18O. During this process, there are four species of 18O-labeled stercobilin that are produced, in addition to the residual unlabeled stercobilin. These four species differ in the number of carboxylic oxygens that underwent the isotopic exchange. For the purpose of a surrogate standard, all experiments are spiked with 2 ppb of stercobilin-18O3 and are normalized to this peak located at m/z 601. Figures 1 and 2 show the typical mass spectra for stercobilin before and after isotopic labeling, respectively.
To determine the minimum volume needed to enrich stercobilin to a detectable volume, a 10-ng/L stock solution of stercobilin in water was prepared. From this stock solution, aliquots of 20, 100, 300, and 500 mL underwent SPE analysis. The purpose of this initial study was to determine the minimum sample volume needed for SPE to obtain a detectable mass spectral signal. Before SPE analysis, all samples were pH adjusted to 9 using ammonium hydroxide and were spiked with 2 ppb stercobilin-18O3 as a surrogate standard. Samples underwent SPE analysis using 1cc 30 meq Oasis MAX cartridges loaded at a flow rate of 1 mL/min. The SPE protocol was as follows: condition with 3 mL of methanol, equilibrate with 3 mL of water, load sample, and elute with 1.2 mL 5% (v/v) formic acid in methanol. Samples were then dried down with nitrogen gas and reconstituted in 1 mL of 20:80 (v/v) acetonitrile–water for analysis. This SPE protocol is based off of previous experiments to determine an optimum SPE analysis methodology. This method yields a percent recovery of 67.0% with a low percent relative standard deviation (%RSD) of 1.2%.
Results and Discussion
Initial SPE results for this experiment are listed in Table I. Using a blank sample that underwent the aforementioned SPE protocol followed by analysis with the FT-ICR mass spectrometer, the minimum detection intensity for stercobilin at [M+H]+m/z 595.3427 is 4.34 × 104 arbitrary units (au). Based on the results of this initial experiment, the 20-mL volume, while still producing sufficient signal above the minimum detection limit, has the lowest %RSD. The higher volumes used for SPE (300 and 500 mL) also show high %RSD values. Most likely the breakthrough volume has been exceeded for these large volumes, and thus some stercobilin is lost during the loading phase. Thus, all subsequent experiments with the swimming pool were performed using a 100-mL sample of pool water. Using this model, a calibration curve was created with concentrations ranging from 1 ppb to 200 ppb in 100 mL of water that then underwent SPE analysis. The calibration curve demonstrated a linear trend with R2 = 0.955, limit of detection (LOD) (3σ) = 1.2 ppb and limit of quantitation (LOQ) (10σ) = 4.1 ppb.
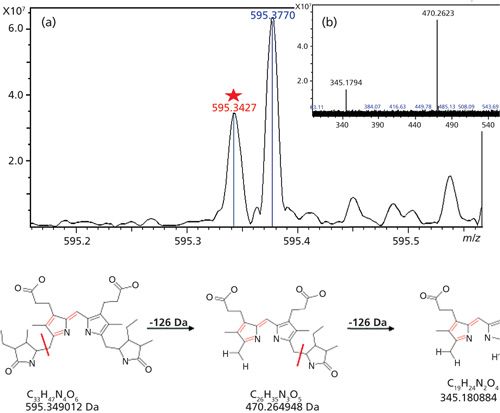
Swimming pool samples were collected from three local western New York public swimming facilities. Each of the three swimming facilities, which will be designated as locations A, B, and C, had both adult and children’s swimming pools. The adult pools have a 250,000-gal capacity and the children’s pools have 30,000–60,000 gal capacities. All swimming pools are filtered using a sand filtration system and are maintained to have an optimum pH of 7.8 and chlorine measurements of 1.2–2.0 ppm. Samples were collected three times throughout the 2014 swim season. The first collection was made on June 16, the second collection on July 19, and the last collection on August 4. Collections were taken from the same location in each swimming pool at the same time of day. SPE analysis was performed on the samples to enrich stercobilin from the samples and then the post-SPE samples were analyzed using FT-ICR MS. Tandem MS-MS was performed on the [M+H]+ 595.3427 Da ion using collision-induced dissociation (CID) at 25 eV with argon as the collision gas to ensure that the peak is indeed stercobilin. Figure 3 is the resulting tandem mass spectrum. Following analysis, typical stercobilin fragment peaks were recorded at 470.2623 Da (mass error, 2.6 mDa) and 345.1794 Da (mass error, 1.4 mDa) (13) indicating that stercobilin was separated and analyzed in the swimming pool sample.
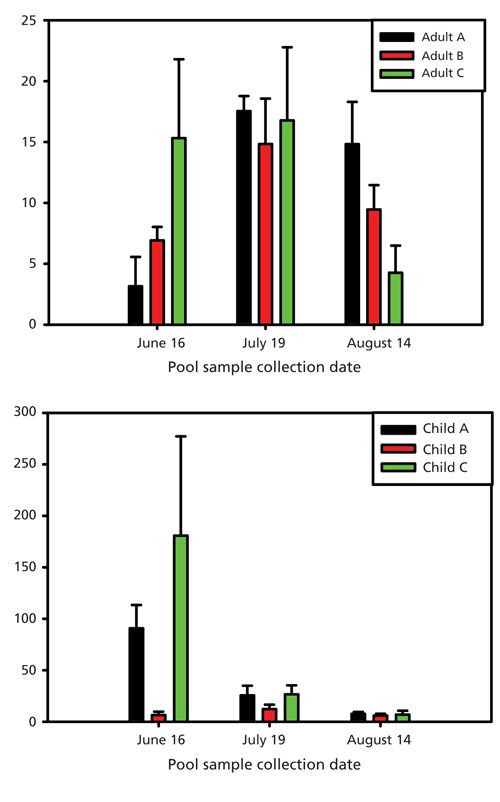
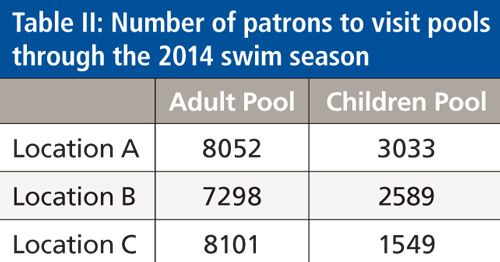
Using the calibration curve, the concentration of stercobilin in pool samples collected in June, July, and August of 2014 was calculated. Figure 4 compares the level of stercobilin found in each pool (adult or children’s) at the three swimming facilities. It was determined that the concentration of stercobilin was found to be higher in that of the children’s swimming pools compared to the adult pools with an overall average of 39.7 ± 60.4 ppb for the children’s pools and 10.2 ± 5.7 ppb for the adult pools. In the month of June, samples were collected immediately after the swimming pools had been filled with water; however, two of the children’s swimming pools (A and C) were not yet treated with chlorine and pH adjusted at the time the samples were collected, with their calculated stercobilin levels at 91.1 ± 21.4 ppb and 183.1 ± 96.7 ppb, respectively. This is most likely the reason for the high concentration of stercobilin found in these pools. Although patrons have not yet begun to swim in the children’s swimming pools A and C, there could be run off from the surrounding area (frequented by pet dogs) that would cause these stercobilin levels to be high. For both the children’s and adult pools, with the exception of the children’s pools A and C in June, the highest levels of stercobilin were measured during July. Table II shows the patron attendance at each pool location throughout the 2014 summer swim season. The measured stercobilin for adult pool versus children’s pools was 15.2 ± 2.0 ppb and 20.4 ± 5.9 ppb, respectively. These data align with patron attendance, which was highest in the month of July, thus the amount of stercobilin exposure would be higher. In August, the stercobilin concentration is less than that of the measured levels in July and June with averages of adult and children’s pool stercobilin concentrations at 8.2 ± 0.9 ppb and 5.5 ± 0.8 ppb, respectively. Again, the data for the lower stercobilin content correspond with the lower patron usage in August.
Conclusion
Initial method development and analysis demonstrate that stercobilin has the potential to be used as a waste biomarker in public swimming facilities. By using SPE analysis, we were able to enrich stercobilin from the swimming pool samples to a detectable and quantifiable signal via mass spectrometry analysis. Additional samples need to be collected and compared to swimming facility records to determine if high levels of stercobilin correlate to their protocols of adjusting pH and chlorine levels. Samples also need to be analyzed after pH and chlorine adjustment to determine if stercobilin levels are then affected in the same manner, demonstrating a safe swimming environment. Studies that correlate E. coli and coliform levels to concentration levels of stercobilin are also planned.
Acknowledgments
We acknowledge the National Institutes of Health for funding that provided the FT-ICR mass spectrometer (S10-RR029517) used here and the Mark Diamond Research Grant for materials and supplies. We would also like to thank the local western New York swimming facilities for granting us access to their pools for this study.
References
(1) “Peeing in The Pool: Olympic Swimmers Cop to the Habit, But Is It Dangerous?” Huffington Post, Available on-line at: http://www.huffingtonpost.com/2012/08/02/pee-in-pool-olympic-swimmers-dangers_n_1733789.html (accessed May 12, 2013).
(2) “Fecal Contamination Found in 58% of Public Pool Samples, CDC Finds” Huffington Post, Available on-line at: http://www.huffingtonpost.com/2013/05/16/fecal-contamination-pool-public-e-coli_n_3287087.html (accessed May 20, 2013).
(3) A. Edney, “Feces Contaminates More Than Half of U.S. Public Pools,” Bloomberg Business, Available on-line at: http://www.bloomberg.com/news/articles/2013-05-16/feces-contaminates-more-than-half-of-u-s-public-pools (accessed May 13, 2013).
(4) A. Sifferlin, “Don’t Drink the Pool Water! It Contains a Surprising Amount of . . . Human Waste.” Time, Available on-line at: http://healthland.time.com/2013/05/16/dont-drink-the-pool-water-it- contains-a-surprising-amount-of-human-waste/?hpt=hp_t3 (accessed May 20, 2013).
(5) C. Hutcheson, R. Cira, S. Gaines, and K. Jones, MMWR62, 385–388 (2013).
(6) “Basics of Recreational Water Illnesses (RWIs).” Available on-line at: http://www.cdc.gov/healthywater/swimming/rwi/rwi-basics.html (accessed February 4, 2014).
(7) “State-Based Healthy Swimming Information,” Centers for Disease Control and Prevention. Available on-line at: http://www.cdc.gov/healthywater/swimming/states.html (accessed March 9, 2015).
(8) “Public Swimming & Bathing Places (Aquatic Facilities) Operational & Biological Contamination Protocol Recommendations.” Available on-line at: www.phila.gov/health/pdfs/PA_Pool_Recs.pdf (accessed Feb 4, 2014).
(9) J.N. Bixler, M.T. Core, B.H. Hokr, J.D. Mason, E. Figueroa, E.S. Fry, V.V. Yakovlev, and M.O. Scully, Proc. Natl. Acad. Sci. U.S.A.111, 7208–7211 (2014).
(10) T.L. Jones-Lepp, J. Environ. Monitor.8, 472–478 (2006).
(11) Y. Miyabara, Y. Sakata, J. Suzuki, and S. Suzuki, Environ. Pollut.84, 117–112 (1994).
(12) Y. Miyabara, J. Suzuki, and S. Suzuki, Environ. Pollut.84, 105–109 (1994).
(13) K.D. Quinn, N.Q.T. Nguyen, M.M. Wach, and T.D. Wood, Rapid Commun. Mass Spectrom. 26, 1767–1775 (2012).
Heather L. Rudolph and Troy D. Wood are with the Department of Chemistry in the Natural Sciences Complex at the University at Buffalo, State University of New York in Buffalo, New York. Direct correspondence to: twood@buffalo.edu

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.