Comprehensive Screening, Confirmation, and Quantification of Organic Pesticides in Foods by GC–MS and LC–MS
Special Issues
This article provides an overview of the instrument platforms, tools, and workflow for analyzing pesticides.
The global focus on keeping food safe has intensified, resulting in major changes in the number of pesticides that are being regulated and monitored, as well as the allowable levels of those pesticides in food. These new regulations drive a need for a wide range of comprehensive and complementary analytical methods and instrumentation that possess the throughput, sensitivity, and breadth of pesticide compound coverage to meet the demand of testing laboratories all over the world. This article provides an overview of the instrument platforms, tools, and workflow for analyzing pesticides.
With the globalization of trade, food production and distribution have become truly international businesses. When we dine out, the fish might come from Japan, the rice from Australia, the spices from China, and the strawberries from Mexico. We take it for granted that the food we eat is safe and free from contamination that could make us seriously ill.
One of the 20th-century advancements that made this cornucopia possible was the development of pesticides. Crops that were previously devastated by pest damage can now be produced at very high yields to meet worldwide demand. However, all pesticide compounds are potentially harmful to humans, and safeguards must be in place to assure that consumers are not exposed to dangerous levels of these pesticides.
Many countries have had regulations regarding the use of pesticides, and their allowable levels in foods, for many years. With the increasing globalization of the food industry, there is greater scrutiny on food safety, resulting in major changes in the number of pesticides that are being regulated and monitored, as well as the allowable levels of those pesticides in food.
A Global Effort to Keep Food Safe
The Food Quality Protection Act (FQPA) of 1996 updated federal regulation of pesticides in the United States to implement a single health-based standard for all pesticides in foods. It provides special protection for infants and children, expedites approval of safe pesticides, and requires periodic evaluation of pesticide registrations and tolerances to ensure that the scientific data supporting registrations will remain up to date in the future. Under this mandate, the US Food and Drug Administration (FDA) performs regulatory monitoring by sampling individual lots of domestically produced and imported foods and analyzes them for pesticide residues to enforce established tolerances (http://www.cfsan.fda.gov/~dms/pes04-06.html). Domestic samples are collected at the point of production, and import samples are collected at the point of entry into U.S. commerce. Products typically are analyzed as the unwashed, whole, raw commodity. The FDA also performs a total diet study (TDS) on four regional market baskets per year, each basket comprising about 300 foods that represent the average U.S. consumer's diet. The foods are prepared as they would be consumed (table-ready) and tested for regulated pesticides. The FDA also performs "focused sampling" that is generally used to follow up on suspected problem areas or acquire residue data on food commodities that are not usually covered during regulatory monitoring. There are more than 1000 registered pesticides in the U.S., and approximately 400 with tolerances established by the Environmental Protection Agency and enforced by FDA.
The European Union (EU) has also recently updated a harmonized regulation of pesticide residues in food, with the new standard taking effect on September 8, 2008 ( http://ec.europa.eu/food/plant/protection/pesticides/index_en.htm). This regulation sets maximum residue levels (MRL) for food and animal feed. The MRL is the highest level of a pesticide residue that is legally tolerated. The regulation covers around 1100 pesticides currently or formerly used in agriculture in or outside the EU. For pesticides that are not specifically mentioned, a general default MRL of 0.01 mg/kg (10 ppb) applies. The EU designates the minimum number of samples to be taken by each member state, and EU reference laboratories coordinate, train staff, develop methods of analysis, and organize tests to evaluate the skills of the various national control laboratories.
Japan also adopted a stricter pesticide regulation in 2006, referred to as the Positive List system, which establishes MRLs for about 500 pesticides ( http://www.mhlw.go.jp/english/topics/foodsafety/positivelist060228/introduction.html ). Produce with pesticide residues exceeding these MRLs or the default tolerance of the "uniform limit" of 0.01 ppm (10 ppb) for those pesticides for which there is no Japanese MRL cannot be marketed in Japan. The regulation also lists 16 pesticides that must not be detected in food. Japan tested more than 200,000 food samples in 2007.
The increased international focus on pesticide contamination in food impacts food producers in virtually any country, since the food market is a global one. Producers in Thailand, for example, must test their products to assure compliance with FQPA, the EU regulation, and the Japan Positive List, if they hope to sell their products in the U.S., Europe, and Japan, which generate much of the world's demand for imported food. These new regulations drive a global need for analytical methods that possess the throughput, sensitivity, and breadth of pesticide compound coverage to meet the demand of testing laboratories all over the world that must ensure compliance.
A Broad Range of Complementary Analytical Methods Is Needed
Food testing laboratories need the ability to detect and quantify hundreds of pesticides, in myriad foodstuffs, at very low levels of contamination. They might be screening for a set of hundreds of known "target" compounds, using a defined test method for each target in the set. Alternatively, a testing laboratory might wish to look for the presence of any pesticide ("unknowns"), without the need for defining a method for each possible compound. They are often called on to do both types of testing, and no single analytical approach can provide the flexibility required to meet this need. Wide variations in the chemical properties of pesticide contaminants and the necessity to detect a very large number of compounds require a range of chromatography and mass spectrometry (MS) systems.
For pesticides that can be vaporized easily without degradation, gas chromatography (GC)–MS (single quadrupole) is an ideal analytical tool, due to the availability of large libraries of pesticide spectra and deconvolution software. This approach can be used to screen, confirm, and quantify both target and unknown compounds. Many foodstuffs, such as garlic and ginger, are very complex, or "dirty," due to the presence of a very large number of background compounds. GC– triple quadrupole MS (QQQ) is very useful for screening, confirming, and quantifying trace-level target compounds in these complex matrices.
Pesticides that are quite polar, not easily vaporized, thermally labile, or not easily derivatized are best analyzed using liquid chromatography (LC) methods. LC–QQQ is particularly useful for analyzing sets of known target compounds. A unique fragment ion for each pesticide is routinely used for screening of each target compound — a second precursor to product ion transition can then be used to confirm the identity of the compounds. The transition with the greatest response is typically used to quantify the pesticides.
Laboratories may be faced with the challenge of determining all pesticides, if any, present in a food sample. LC–time of flight MS (TOF) screening methods provide extremely accurate molecular mass information (typically < 2 ppm) that can be searched against exact mass databases of thousands of compounds. Compounds found in this type of screen can be confirmed and quantified using LC–MS-MS analysis on either QQQ or quadrupole time-of-flight (Q-TOF) instruments.
Analyzing for Target Pesticides
One of the most common activities in a food-testing laboratory is analysis for a set of target pesticides of specific interest. A finite set of pesticides might be used for production of a particular food crop, or a specific set might be especially toxic to children, necessitating the routine analysis for those compounds. GC–MS (single quadrupole) utilizing deconvolution software has broad applicability for this type of analysis. The Automated Mass spectral Deconvolution and Identification System (AMDIS) was developed by the National Institute of Standards and Technology, and is incorporated into deconvolution reporting software (DRS) available from Agilent Technologies. Deconvolution separates unresolved peaks at any given retention time into sets of "cleaned" component spectra that can be matched to library spectra. The analyst runs the method that was used to generate the Pesticide and Endocrine Disruptor Database Library and locks the method, so that retention times will match those stored in the database. DRS then uses deconvolution and locked retention times to identify pesticides from the database.
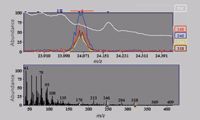
Figure 1: GCâMS analysis of p,p'-DDE in spinach. Data were generated using a J&W DB-5ms column, a model 7890A GC system, and a model 5975C MSD mass spectrometer (Agilent Technologies).
This is particularly useful in the analysis of extracts from foods such as spinach (Figure 1). In this case, one of the pesticides of interest is p,p'-dichlorodiphenyldichloroethylene (p,p'-DDE), a breakdown product of DDT. The total ion chromatogram (TIC) does not show a discernible peak at the p,p'-DDE retention time (24.07 min). Deconvolution analysis found 768 different components over the whole TIC. After a pesticide library search, 12 of these had a match, including p,p'-DDE. The black ions in the bottom window of Figure 1 represent the raw scan, before deconvolution. The white ions are a deconvoluted component that matches the known spectrum of p,p'-DDE. Plotting the extracted ion chromatograms for this component (246, 248, 316, and 318) in the top window of Figure 1 also helped to confirm the presence of the p,p'-DDE peak in the TIC. Figure 2 illustrates the extraction of the p,p'-DDE spectrum from the raw spectrum, and its match to the library spectrum, resulting in a positive confirmation of the presence of this pesticide in the spinach sample. The use of powerful integrated deconvolution software such as DRS, in combination with specialized database libraries such as the Japanese Positive List Pesticide Library (1) or the more comprehensive Pesticide and Endocrine Disruptor Library (2) (Agilent Technologies, Palo Alto, California), can increase the efficiency of a food testing laboratory greatly. These GC–MS methods can screen for hundreds of compounds and quantify a subset of them in as little as 2 min after the run using DRS. With traditional GC–MS data processing, an analyst would need as much as 15 min per sample to review the data and confirm pesticide hits.

Figure 2: Confirmation of p,p'-DDE in spinach using DRS and the Pesticide and Endocrine Disruptor spectral library (Agilent Technologies).
LC–MS methods are also very useful for analysis of target pesticides that are too polar or thermally labile for GC–MS. LC–QQQ is especially suited to very complex food matrices that can interfere with the analysis.
Dealing with Dirty Sample Matrices
Sample preparation is a significant challenge with food analysis and makes up a considerable portion of the total pesticide analysis time. Robust analysis methods that can tolerate very complex sample matrices without loss of sensitivity or accuracy are a must to reach throughput goals.
Multiple reaction monitoring (MRM) with a QQQ system provides detection of trace amounts of target compounds in complex matrices, providing screening, confirmation, and quantification in one analysis (although a replicate analysis is usually required in most laboratories for confirmation). The precursor ion of interest generated by the source is selected and isolated in the first (Q1) quadrupole. A hexapole collision cell (Q2) fragments the molecule, and the product ions are selected in Q3. By choosing product ions from this transition that are characteristic for the pesticides of interest, chemical noise is separated from signal, providing very high sensitivity and selectivity, even in very dirty matrices. With careful selection of highly specific product ions for each pesticide, it is possible to create an MRM assay that can be used to identify and quantify hundreds of pesticides in a sample simultaneously.
Efficient use of LC–QQQ MRM methods requires optimization of a few parameters, including voltages on the fragmentor and the collision cell. Parameters must be optimized to generate the most intense product ions representative for each target compound, and automated method optimization software simplifies this task enormously.
LC–QQQ is an excellent platform for screening, confirmation, and quantification of pesticides. A screening method uses one transition per compound to look for a large number of pesticides on the target list. Confirmation of pesticides detected in the screening is done using two transitions per compound. For example, 301 pesticides have been rapidly identified in one analysis with most having a limit of detection (LOD) of 0.01 mg/kg (10 ppb), which is the MRL for baby food in the U.S. and the general default MRL for foods in the European Union. These levels were reached in a single analysis using positive ion electrospray with 99 transitions per time segment, and a quantifying and qualifying ion for each compound (3). Two types of vegetables (pepper and tomato) were evaluated, and a linearity of response over three orders of magnitude was shown for quantification (r 2 > 0.99; Figure 3).

Figure 3: Calibration curve for diazinon in pepper using a six-point curve from 0.1 to 100 ng/mL with a linear fit and no origin treatment. Data were generated using a ZORBAX SB-C-18 1.8-µm LC column with a model G6410A QQQ system in the positive ESI mode (Agilent Technologies).
GC–QQQ also provides sensitive and reproducible screening, confirmation, and quantification of pesticides that are not highly polar, particularly in very complex matrices such as garlic and ginger. Despite the high background of these matrices, pesticide compounds can be detected at levels as low as 0.5 ppb, with very high signal to noise and peak area precision as good as 6% RSD (Figure 4). For certain "active" pesticides such as acephate, the use of chemical protectants can reduce adsorption of analytes during the analysis, increasing sensitivity and improving peak shape. The result can be a marked improvement in accuracy of quantification in complex matrices (4).
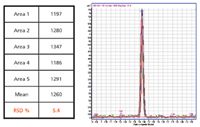
Figure 4: Precision of GC-QQQ quantification of 500 fg of cyanophos spiked in garlic. Data were generated using an HPâ5ms UI (Ultra Inert) 30 m x 0.25 mm, 0.25-µm film thickness GC column and a model 7000A QQQ MS system (Agilent Technologies). The pesticide cyanophos was spiked into a garlic matrix at 0.5 ppb and replicate analyses performed at 500 fg on-column. The peaks are superimposed for the five runs, with the replicate peak areas, mean peak area, and RSD listed in the table to the left.
Backflushing the GC column, rather than using oven bakeout, also removes many high-boiling interfering substances in the sample (Figure 5) without altering retention times in subsequent runs or shortening the life of the column (5). Backflushing improves data quality while shortening cycle time and increasing column life. It also eliminates ghost peaks and reduces ion source contamination by keeping excess column bleed and heavy residues from being introduced into the mass spectrometer.
One key to the successful rapid analysis of large numbers of pesticides is the dwell time of the QQQ mass spectrometer — the time spent to measure the signal of any one precursor to product ion transition. The shorter the dwell time, the more transitions that can be analyzed per unit time, and the more pesticides that can be quantified per unit time.

Figure 5: Backflushing the GC column for higher signal to noise and shorter cycle times. Comparison of backflushing using capillary flow (red chromatogram) versus column bake-out (blue chromatogram) utilizing a model 7890A GC system and an HP-5ms GC column (Agilent Technologies).
Analyzing for Unknown Pesticides
Often, food-testing laboratories want to determine the entire spectrum of pesticides that might be present in a sample, rather than just a limited set of target compounds. While GC–MS using deconvolution is an effective tool to screen for over 900 pesticides (2), LC–TOF or LC–QTOF can be used to screen for any number of LC–MS-amenable compounds.
TOF and QTOF are extremely accurate MS techniques, routinely achieving <2 ppm mass accuracy. Analysis software such as the Molecular Feature Extractor (MFE) from Agilent Technologies can find all ions representing real compounds in the sample. Noise and other extraneous ions are excluded. The resulting list of masses is then searched against a database of theoretical exact masses of compounds based on their molecular formulas and selected adducts, to identify pesticide compounds (Figure 6). This method has been used to screen food extracts for 600 pesticides and their degradates. The limit of detection (LOD) was determined for 100 of the 600 pesticides, and varied from <0.01 mg/kg (10 ppb) for 34% of the compounds, to <0.5 mg/kg (500 ppb) for 95% of the compounds (6). Using the MFE algorithm streamlines the data analysis for screening of hundreds of compounds at sensitive levels to minutes, compared with hours or even days using a manual approach. TOF can be used to identify an unlimited number of ionizable compounds, and the sensitivity is not impacted by the number of compounds screened. While LC–TOF can provide screening and quantification of both unknown and targeted pesticides, LC–QTOF or another MS-MS approach is required to confirm identification.

Figure 6: Pesticide screening for unknowns using LCâQTOF. The top panel shows the full spectrum total ion chromatogram (TIC) using a model 1200 LC system, a ZORBAX Eclipse XDB, 5-µm column, and a model 6510 LCâMS QTOF system (Agilent Technologies). The Molecular Feature Extractor found 510 compounds in the TIC, 15 of which had hits from the EXACT MASS database search (bottom panel). The three highlighted compounds were further confirmed by MSâMS analysis.
Conclusion
One need only look to recent events to appreciate the importance of pesticide testing in food. A series of poisonings in Japan in December of 2007 and January of 2008 that sickened 10 people was linked to methamidophos pesticide contamination of gyoza dumplings imported from China. The impact on Japanese food buying habits was immediate, as importation of Chinese vegetables fell 40% shortly after the source of the poisoning was determined.
In another example, pet food vegetable proteins contaminated with melamine were imported into the United States from China in early 2007, resulting in the deaths of several cats and dogs. More recently, the same contaminant has appeared in baby formula in China.
Food-testing laboratories require a broad range of complementary MS-based analytical technologies and methods to screen, confirm, and quantify the presence of as many as 1000 pesticide compounds. Screening methods can be used to detect as many contaminants as possible in a single run, and these methods don't necessarily have to quantify all the compounds. The screening method can be followed by target compound analysis that is very selective, sensitive, and quantitative. For LC-amenable pesticides, this means running LC–TOF or LC–QTOF followed by LC–QQQ. For GC-amenable pesticides, this means running GC–MS with DRS for screening followed by GC–QQQ for target compound analysis at low levels. The method used by any given laboratory is determined by its specific pesticide analysis goals, existing instrumentation, the budget for acquisition of new instrumentation, and required LODs.
When choosing new instrumentation, laboratories doing pesticide testing also should consider whether a particular manufacturer has a complete solution that includes all of the above platforms, and whether those platforms share common, interchangeable ion sources, a unified software platform, and transferable collision voltages, thus providing a uniform workflow. The robustness of a manufacturer's systems is a key factor, including the tolerance of the ion source to contamination and its required frequency of cleaning and maintenance. Utility of the software and its integration with comprehensive pesticide spectra libraries and exact mass databases are also keys to success. Finally, the manufacturer's reputation for excellence in GC and LC–MS instrumentation should be considered.
References
(1) P.L. Wylie, Screening for pesticides in food using the Japanese Positive List Pesticide Method, Agilent Application Note 5989-7436 (2007).
(2) P.L. Wylie, Screening for 926 Pesticides and Endocrine Disruptors by GC/MS with Deconvolution Reporting Software and a New Pesticide Library, Agilent Application Note 5989-5076 (2006).
(3) J.A. Zweigenbaum, Multiresidue analysis of 301 pesticides in food samples by LC/Triple Quaduprole mass spectrometry, Agilent Application Note 5989-8614 (2008).
(4) M. Anastassiades et al., J. Chromatogr., A 1015, 163–184 (2003).
(5) C.-K. Meng, Improving Productivity and Extending Column Life with Backflush, Agilent Application Note 5989-6018 (2006).
(6) J.A. Zweigenbaum, Automated screening of 600 pesticides in Food by LC/TOF MS using a molecular–feature database search, Agilent Application Note 5989-5496 (2006).
Philip Wylie, Jerry Zweigenbaum, Melissa Churley, Chin-Kai Meng , and Cao Zhe are Senior Applications Chemists, Agilent Technologies, Santa Clara, California.

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.