Effective Abatement of High Lead Level Contamination in a Forensic Firing Range via Inductively Coupled Plasma–Mass Spectrometry
Test firing a firearm is frequently used for forensic firearms and bullet identification. Airborne lead-containing particles are emitted when a firearm is tested, leading to lead building up on surfaces, exposing employees to potential lead-related health risks. Prior to cleaning, lead surface concentrations in the firing range at the National Forensic Laboratory Services in Ottawa were found to be higher than the Environmental Abatement Council of Ontario (EACO) post-abatement limit, with the highest level 56 times the limit. Inductively coupled plasma–mass spectrometry (ICP-MS), along with internal standardization, revealed that wiping surfaces with either a commercial decontamination product containing ethylene glycol butyl ether (EGBE) or alcohol cleaning pads satisfied the EACO standard by removing over 90% of lead from test surfaces whereas an external cleaning company only removed 36% of lead from the same surfaces. Fortunately, lead cross-contamination was minimal outside the firearms section and well below the residential EACO limit.
Conventional gunshot residue (GSR) found after firearm discharge consists of particles composed primarily of lead, antimony, and barium (1). Lead styphnate (C6HN3O8Pb) is often used as the primary explosive in conventional ammunition primers and, after firing, yields airborne GSR particles containing lead (1–3). However, lead bullets or pellet projectiles produce larger quantities of airborne lead metallic particles upon discharge (1,4). Lead bullets also deposit thin layers of lead inside the barrel of a firearm, and even if a jacketed bullet is subsequently fired using the same firearm, residual lead inside the barrel is still emitted upon discharge (5). Because lead is a soft and low-melting-temperature metal, because of the energy and heat associated with terminal ballistics, lead bullets expel airborne molten lead particles upon impact (5,6).
The discharge of a firearm can easily coat the user’s hands and face with lead particles (7). Without proper handling and lead decontamination, firearm users expose themselves to potential health risks, particularly lead poisoning (4). Lead poisoning can cause a variety of health effects in adults, including but not limited to nervous system damage, abnormal sperm in men, and miscarriages in women (8). Lead poisoning is concerning for children because it can severely affect their mental and physical development with irreversible changes (9,10). Even with modestly elevated blood–lead levels, breast-feeding or pregnancy can directly affect a nursing baby or fetus, respectively (11,12). Lead toxicity may remain asymptomatic until a large concentration of lead has accumulated, which could prove fatal if left untreated (8).
GSR accumulation in firing ranges can result in high lead concentrations on surfaces, exposing personnel to potential health risks (4). Firing ranges, such as the one located at the Royal Canadian Mounted Police (RCMP) National Forensic Laboratory Services in Ottawa (NFLS-O), are purposed for forensic firearms and bullet identification or association. Although the NFLS-O firing range sees fewer firearm discharges per week than a recreational or training firing range, high concentrations of lead were still observed there. Improper handling and cross-contamination could lead to personnel outside the range also being exposed through secondary or tertiary transfer (13). Proper cross-contamination prevention, such as removing contaminated work clothes before exiting the range, can reduce secondary and tertiary transfers of GSR (4).
For a surface to be considered “lead-free,” the Environmental Abatement Council of Ontario (EACO) sets a limit of 0.215 µg/cm2 for the post-abatement residual lead surface concentrations in firing ranges, and 0.043 µg/cm2 for residential areas where sources of lead are not handled (14). A preliminary analysis by an external company using inductively coupled plasma–optical emission spectrometry (ICP-OES) found surface lead concentrations in the NFLS-O firing range exceeded the EACO limit by sevenfold.
The United States Environmental Protection Agency (EPA) recommends trisodium phosphate (TSP) for removing particles of lead-containing paints in residential homes because it selectively chelates lead (15). D-Lead, a commercial product containing ethylene glycol butyl ether (EGBE), supposedly acts similarly to TSP by chelating lead. The supplier, ESCA Tech Inc., claims D-Lead can reduce 322 μg/cm2 (149,767% of the EACO limit) down to 0.04 μg/cm2 (19% of the EACO limit) of lead on a window glass pane (16). For cleaning hard surfaces, the frequency, duration, and thoroughness of the cleaning may be more important than the choice of a lead-specific chelating detergent, as will be demonstrated below.
However, considering that a firing range contains porous surfaces, choosing a decontaminant product that can chelate lead effectively is vital. Indeed, a study compared TSP to a detergent having a low phosphate concentration and to suction through a high efficiency particulate air filter and found inconsistency of cleanliness post-abatement between all methods of cleaning (17).
Two methods have been used to collect dust samples for heavy metal analysis—vacuuming and wiping. Vacuuming has mostly been used for household dust studies, where cross-contamination between different surfaces was not a concern (18). In fact, a vacuum was used to obtain a consistent composite sample of fresh dust from a whole house, which was then digested prior to analysis using ICP-OES or inductively coupled–mass spectrometry (ICP-MS), depending on analyte levels (18). In contrast, the wiping approach was used to assess localized surface contamination (15). To assess lead contamination inside and outside a firing range, a technique providing a low detection limit and a wide linear dynamic range like ICP-MS is desired to measure both the trace lead levels outside the firearms section and the higher levels in the range itself.
The goals of this study were to develop an ICP-MS method and use it to do the following: (a) Assess the levels of lead contamination in a forensic firing range and adjacent work areas designated for firearms and bullet identification, (b) assess cross-contamination outside the firearms section, and (c) compare three cleaning methods for effective lead abatement by i) an external cleaning company using commercial degreasers, ii) EGBE-containing wipes, and iii) isopropanol cleaning pads. Surfaces tested included areas where lead was least expected (such as office desks), areas where some lead was expected (such as display tables near the firearms laboratory section), and contaminated areas (in particular, inside the firing range). To our knowledge, this is the first study where ICP-MS was used to assess contamination in and around a firing range in close proximity to lead-free facilities with no safety precautions other than the ones put in place for the firearms staff.
Experimental
Instrumentation
A NexION 300D quadrupole ICP-MS instrument (PerkinElmer Inc.) was used, which features a triple cone interface consisting of nickel (Ni) sampler and skimmer cones along with an aluminum (Al) hyper-skimmer cone. This ICP-MS instrument was equipped with a quartz torch and injector, a quartz cyclonic spray chamber, a PFA-ST microflow nebulizer connected to a model SC-2 DX autosampler with a Fast DXi module equipped with a 300 μL perfluoroalkoxy alkanes (PFA) sample loop (Elemental Scientific Inc.). The operating conditions are as follows: 18 L/min argon plasma gas; 1.00 L/min argon auxiliary gas; 1.06 L/min nebulizer gas; 0.10 mL/min sample uptake rate; and 1.40 kW radio-frequency power. They were optimized by the instrument’s software while aspirating a 1 µg/L multielement solution. The measurement conditions (50 ms dwell time in pulse counting mode, with 40 sweeps per replicate during 24 s) were selected to allow six replicate measurements of 103Rh+ and 208Pb+ during the signal plateau resulting from a 300-μL injection (see below).
Images of lead distributions in the dust standard reference material (SRM) and in particles from the firing range were obtained using a Vega3XMU scanning electron microscope (SEM) (Tescan) with an energy dispersion spectrometer (EDS) (Oxford Instrument x-Max 50) operating with a current of 89.75 µA at 20 kV. Conductive carbon tape was used to mount samples on a 0.5 inch aluminum stub (Semoptics Ltd.). Samples were then graphite-coated using a Carbon Coater 108 Carbon/A (Cressington Scientific Instruments UK) for better imaging.
Reagents, Certified Reference, Materials, and Samples
A MilliQ system (Merck KGaA) provided doubly deionized water (DDW) (18.2 MΩ cm). Optima grade HNO3 and HCl (Fisher Scientific) were used for digestion of the samples and for their dilution. Glassware was washed with trace metal grade HNO3 (Fisher Scientific). A dilution of a 1000 mg/L Rh in 20% HCl Specpure plasma standard solution (AlfaAesar) was used for internal standardization. Standard additions were made with a dilution of a 10,020 mg/L lead in 5% HNO3 Specpure plasma standard solution (AlfaAesar). Dust SRM 2584 was obtained from the National Institute of Standards and Technology (NIST). All samples were collected in the RCMP NFLS-O facility in Ontario, Canada.
Sampling Procedure
Following a study on lead surface wipe analysis (15), a 14 cm by 14 cm square stencil created using painter’s tape was measured out using a 14 cm paper ruler, which was discarded after each sampling surface to prevent cross contamination. A 70% (w/w) isopropanol (alcohol cleaning) pad (Personal Respirator Cleaning Pad, Allegro Industries) was used to scrub across and down the stenciled surface. The alcohol pad was then placed into a sterile 50-mL polypropylene vial, sealed, and labeled. To test the effectiveness of cleaning methods, stenciled areas were cleaned with either D-Lead wipes soaked in EGBE or alcohol cleaning pads; then, the same surface was sampled using a new alcohol cleaning pad. Because of the nature of the lead contamination related to firearms discharge in the range, it was impossible to do multiple replicates of each surface. Table I shows that only one replicate was done for real samples with six measurement replicates per analysis.
Leaching Procedure
The wipes or cleaning pads were leached for lead, as described in the literature (15). Each wipe or cleaning pad loaded with the sample was removed from the 50-mL vial using tweezers. It was cut into roughly 1 cm × 1 cm square pieces using scissors and placed flat at the bottom of a 50-mL polytetrafluoroethylene (PTFE) beaker. After air-drying for 15 min to remove most of the isopropanol, 10 mL of DDW was pipetted into the beaker, which was covered with a watch glass and heated at 125 ˚C for 5 min on a hotplate. Then, 2 mL of HCl followed by 2 mL of HNO3 were pipetted into the beaker, which was covered with a watch glass and heated at 125 ˚C for 30 min. The beaker was then taken off the hotplate to cool in the fume hood with the watch glass on. The solution was decanted and poured into a 50-mL glass volumetric flask. The watch glass and sides of the beaker were rinsed with DDW and the wipe or pad was squeezed with tweezers to recover as much analyte as possible before diluting to volume. The leachates were finally centrifuged (Eppendorf Centrifuge 5702) at 4400 rpm for 30 min and decanted again to reduce the amount of particulates in solution that might clog the nebulizer.
Calibration Strategy
Semi-quantitative analysis of sample solutions was done to enable adjustment of their concentrations to 10–20 µg/L prior to standard addition. All diluted samples contained 5% (v/v) HCl and 5% (v/v) HNO3. Standard additions of 10, 20, 30, and 40 µg/L lead were made to each sample or SRM, and 1 µg/L Rh internal standard was included in all solutions. SRM 2584 dust served as quality control to verify the accuracy and precision of the method.
Results and Discussion
Possible Interferences
Table I shows that the potential 206Pb++ interference on 103Rh+ was negligible at the dilution factors used to keep the lead concentration in sample solutions at around 10 µg/L, similar to the amount of lead in each standard addition. The potential 86Sr17O+ and 87Sr16O+ interferences on 103Rh+ were similarly negligible because, even in the SRM, the strontium concentration of 0.16 μg/L after dilution was much lower than that of rhodium. Furthermore, the oxide level (for example, CeO+/Ce+) was optimized to be below 5% while aspirating a 1 µg/L multielement solution.
Calibration Strategy
An internal standard was required to compensate for occasional gradual clogging of the nebulizer by residual particulate matter, and rhodium was selected because it is not likely to be at levels affecting the calibration in the range. Although more similar in ionization potential and mass to lead than rhodium, bismuth could not be used because it is present in bismuth-made projectiles found in the range (19) and at 9 mg/kg in SRM 2584. The method of standard addition was thus used to compensate for different matrix effects from the cement, wood, and steel surfaces sampled. In general, the square of the correlation coefficient for the calibration was better than 0.99. The detection limit, calculated as three times the standard deviation of the blank signal divided by the slope of the calibration curve for SRM 2584, was 0.1 µg/L.
Lead Recovery with Proposed Method
NIST dust SRM 2584 with 0.9761 ± 0.0067% (m/m) lead was first used on its own to test the leaching method. The concentration found, 0.950 ± 0.025% (m/m) lead (n = 3), demonstrates that most of the lead was leached. Next, the dust was folded into an alcohol cleaning pad to test whether the pad interfered with leaching of lead. An identical result of 0.950 ± 0.023% m/m lead (n = 3) was obtained, demonstrating that the wipe does not interfere with the leaching method. Finally, 5 mg of dust on wax weighing paper was sprinkled onto a smooth clean surface measuring 14 cm by 14 cm, and the area was then wiped following the sampling procedure. This yielded 0.890 ± 0.048% m/m lead (n = 3). Clearly, the sampling method deteriorated analyte recovery, with the measured concentration corresponding to 85–96% recovery. This is in agreement with the 80–120% recovery range in a one-wipe composite study; where the recovery largely depended on the dust loading of the cleaning pad or wipe, some mass loss being expected (15).
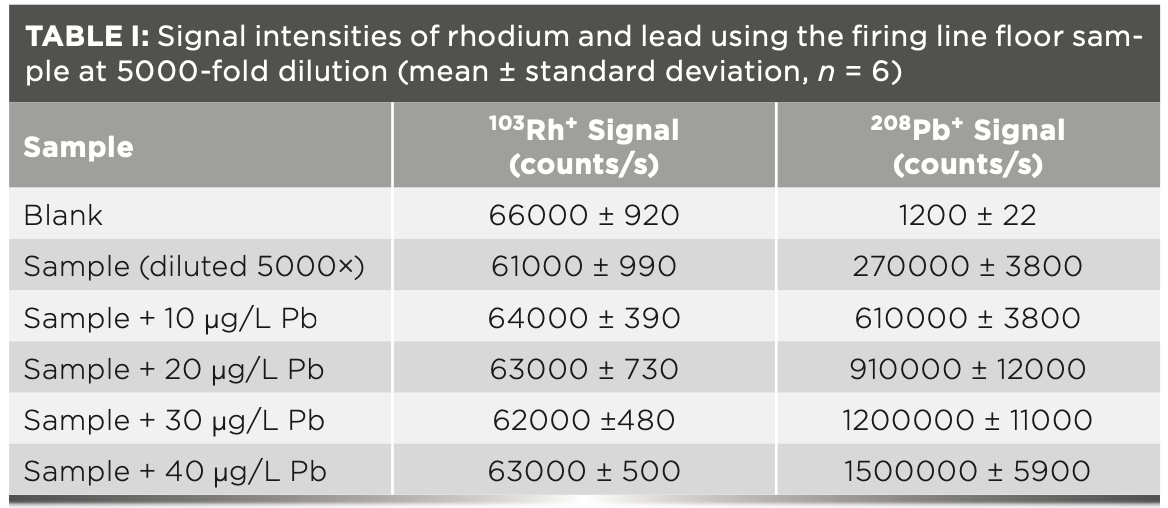
Lead Cross-Contamination in the Firearms Section Outside the Range
The firearms section is divided into separate working areas, including the firing range, examination laboratory, microscope rooms, and workshop. The range is the only area where externally hired cleaners were employed to abate lead. Firearms are only discharged in the range, causing the highest buildup of lead species over time. Other working areas are used for examination and cleaning of firearms, which will cause lead cross-contamination. For example, cleaning the barrel of a firearm will expel residual lead from previous discharges. Figure 1 shows that cross-contamination did not exceed the EACO threshold in some areas of the firearms section. Other locations in the workshop area where firearms are disassembled and cleaned have lead concentrations over the EACO limit. In particular, the sink counter had a surface lead concentration over three times the EACO limit. This sink counter is where personnel wash their hands of lead and would be a point of potential cross-contamination to other areas within the forensic laboratory external to the firearms section.
Figure 1: Concentration of lead on surfaces in the firearms section, excluding the range. The standard deviations are too small to appear on the graph.
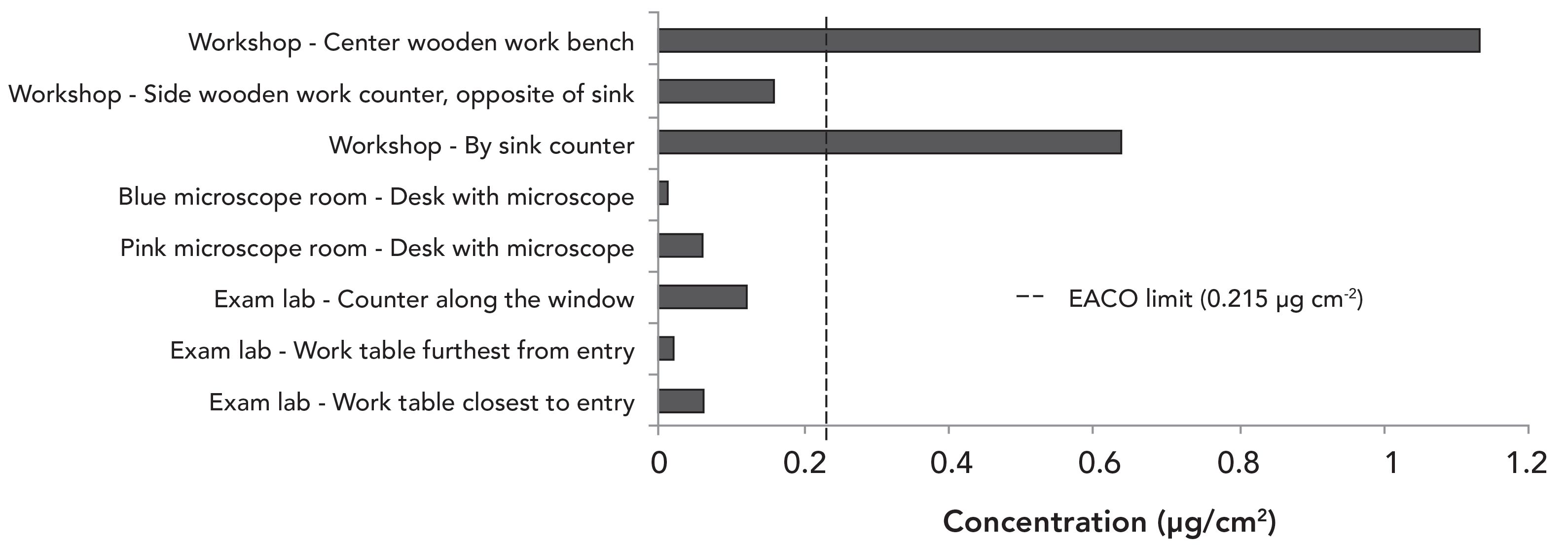
Lead Contamination in the Firing Range
Samples were taken on multiple surfaces before cleaning and again right after the cleaners completed their work. The post-abatement samples were taken as close as possible to the spots that were sampled before cleaning. Figure 2 shows that the lead concentration remained several folds above the EACO post-abatement limit. There were several areas where the lead concentration increased after cleaning by the external company: wall surfaces near the ceiling, wall surfaces near the firing line, the floor near the water tank, the side panel of the water tank bullet catch, and the top panel of the bullet catch. Marks left on the stainless-steel surfaces (both top and side) of the water tank bullet catch (Figure 3) post-abatement as well as remaining high lead concentrations on the surface suggest that the cleaners did not remove lead but merely spread it around. Because lead is a relatively soft metal, it is highly possible that the marks on the surfaces of the tank are caused by lead being smeared onto the surface.
Figure 2: Concentration of lead on surfaces in the range. Samples were collected before and after the external cleaning company finished cleaning (n = 3).
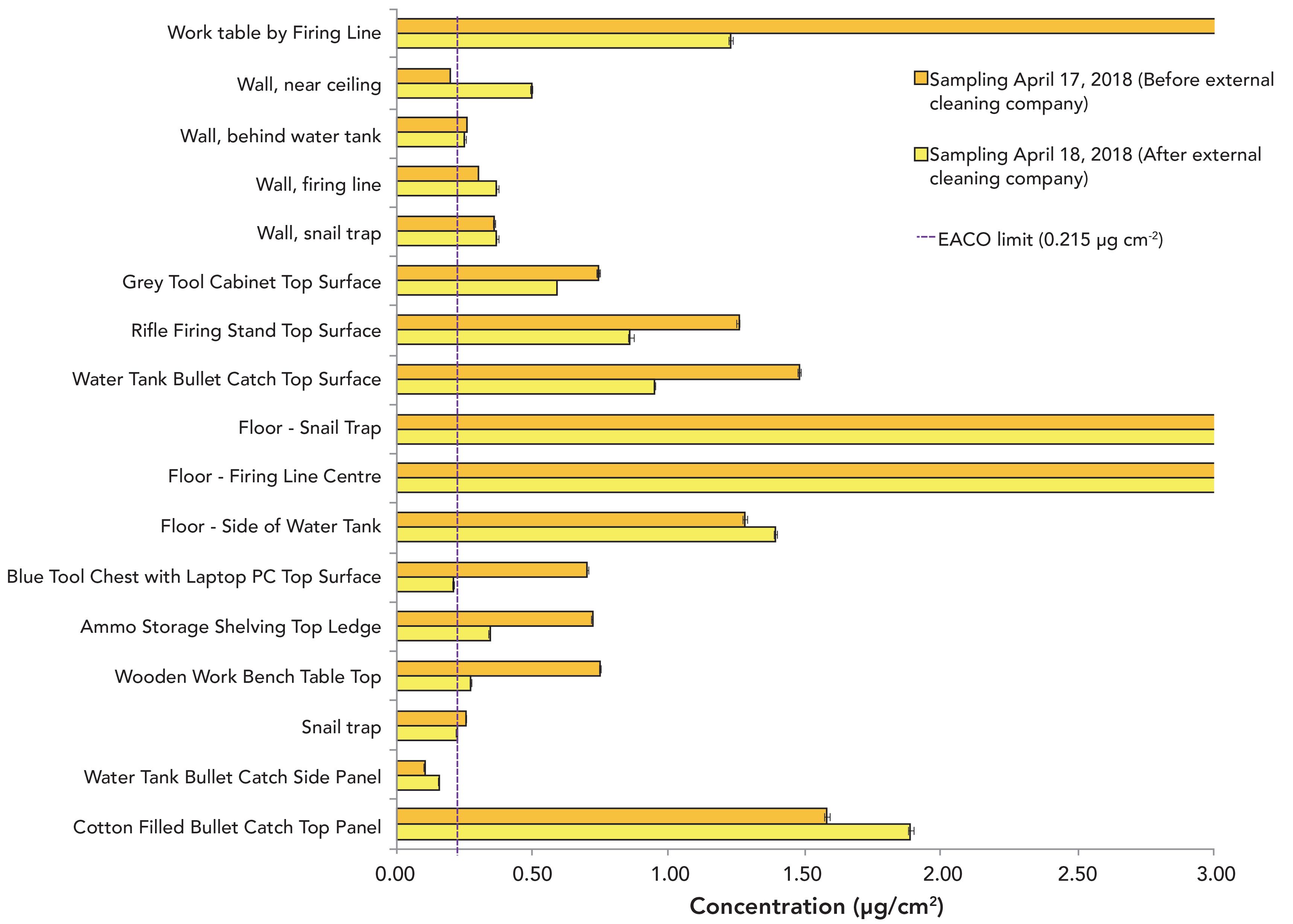
Figure 3: Photographs of the stainless-steel surface of the water tank bullet catch top panel (top) and side panel (bottom) after decontamination by the cleaners, where smear marks (indicated by the red circles) are evident.

Efficiency of Other Cleaning Methods
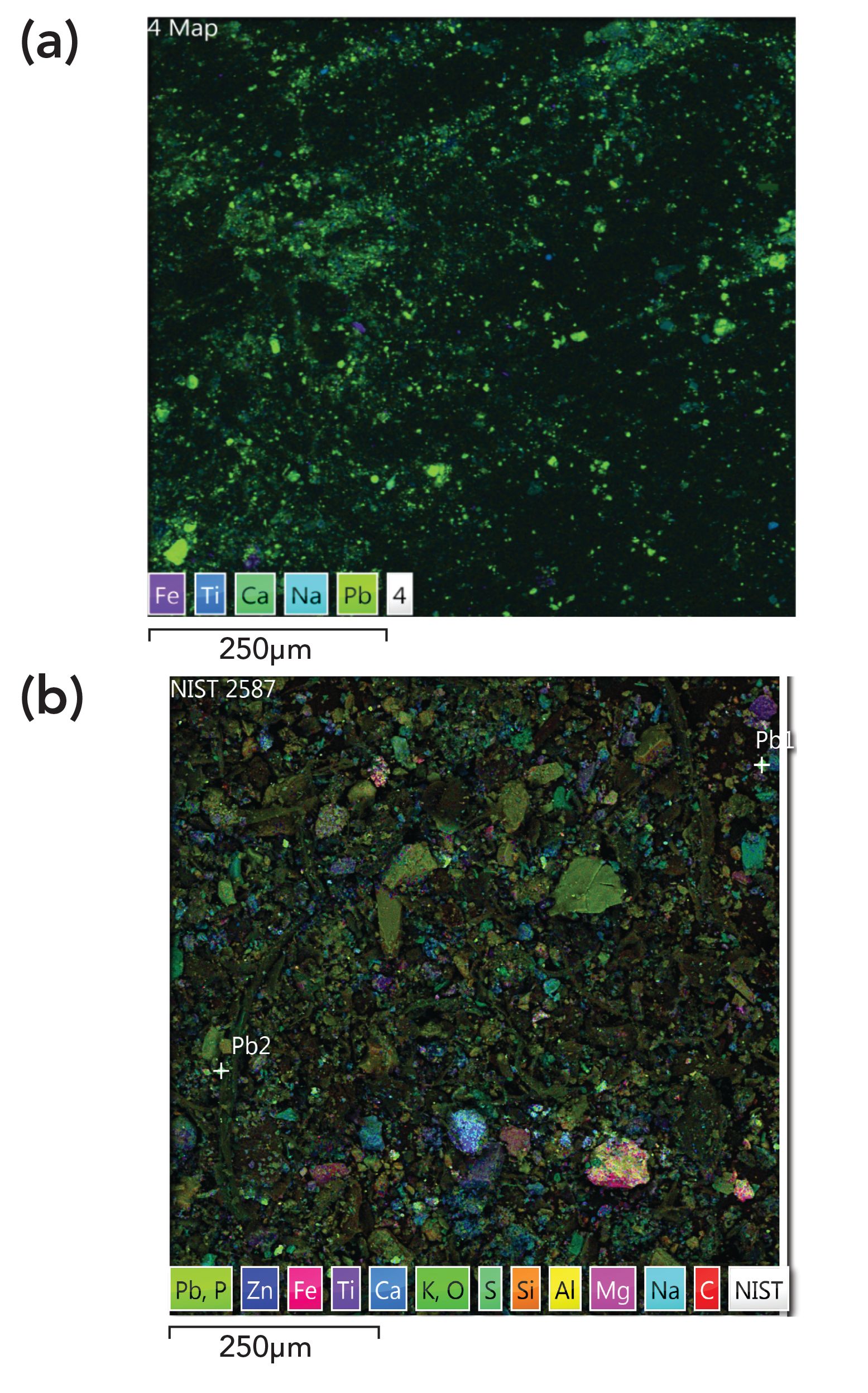
To determine if the lead concentrations in the range could be brought down to acceptable levels using commercial products, the NIST dust SRM was not suitable because the range contains GSR particles and other lead species, but the SRM dust, which was collected from residential homes, does not (Figure 4). Previous speciation analysis of SRM 2584 indicated that 98.5% of lead is present as carbonate and 1.5% as sulfate (20). In contrast, Figure 4 shows that lead in the range is in a different form than in the dust SRM. The effectiveness of the different cleaning methods was thus assessed only in the range itself.
Figure 4: SEM-EDS images (magnified 924-fold) of (a) particles in the range, containing mostly Pb metal (green spots), and (b) dust SRM containing different Pb species.
Because lead concentration could be highly variable over a large surface area, homogeneity of coverage was verified over a small surface. For instance, the lead concentration was tested on the stainless steel top of the water tank bullet catch within two square areas, standardized by measuring with stencils laid side by side. The lead concentration was 0.950 ± 0.003 μg/cm2 in the first square and 0.950 ± 0.006 μg/cm2 in the second. To similarly assess the efficiency of the different cleaning methods, two square stencils were likewise laid side by side for pre-abatement and post-abatement sampling, respectively. Lead concentration during pre- and post-abatement was tested on two surfaces: (1) the floor near the firing line and (2) the stainless-steel surface of the top panel of the water tank. The top was chosen over the side purely because of ease of wiping the surface and the larger surface area.
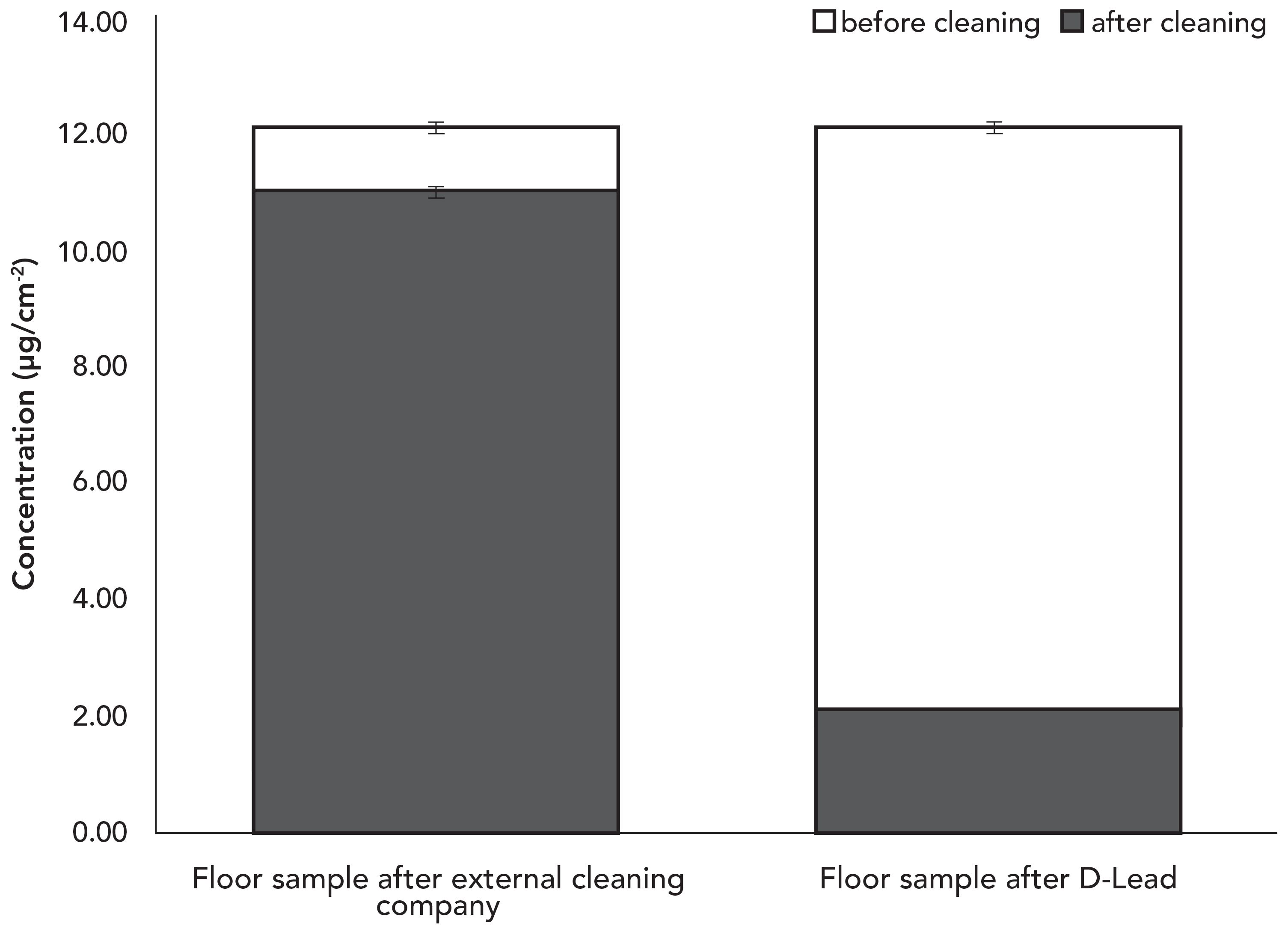
Because the floor near the firing line is painted concrete, its porosity made wiping all residual lead from the floor more difficult than expected. Figure 5 shows that the external cleaning company only removed 9% of lead from the surface, leaving behind 11.01 µg/cm2 or 51 times the EACO limit. The post-abatement concentration found after using an EGBE decontaminant product was 2.11 µg/cm2, indicating that 83% of the lead was removed from the same surface. Unfortunately, the remaining lead concentration was still 9.8 times the EACO limit despite the greater removal efficiency using a decontaminant product containing EGBE.
Figure 5: Surface Pb concentration on the floor near the firing line before and after cleaning by the external cleaning company and a commercial EGBE-containing lead decontamination product.
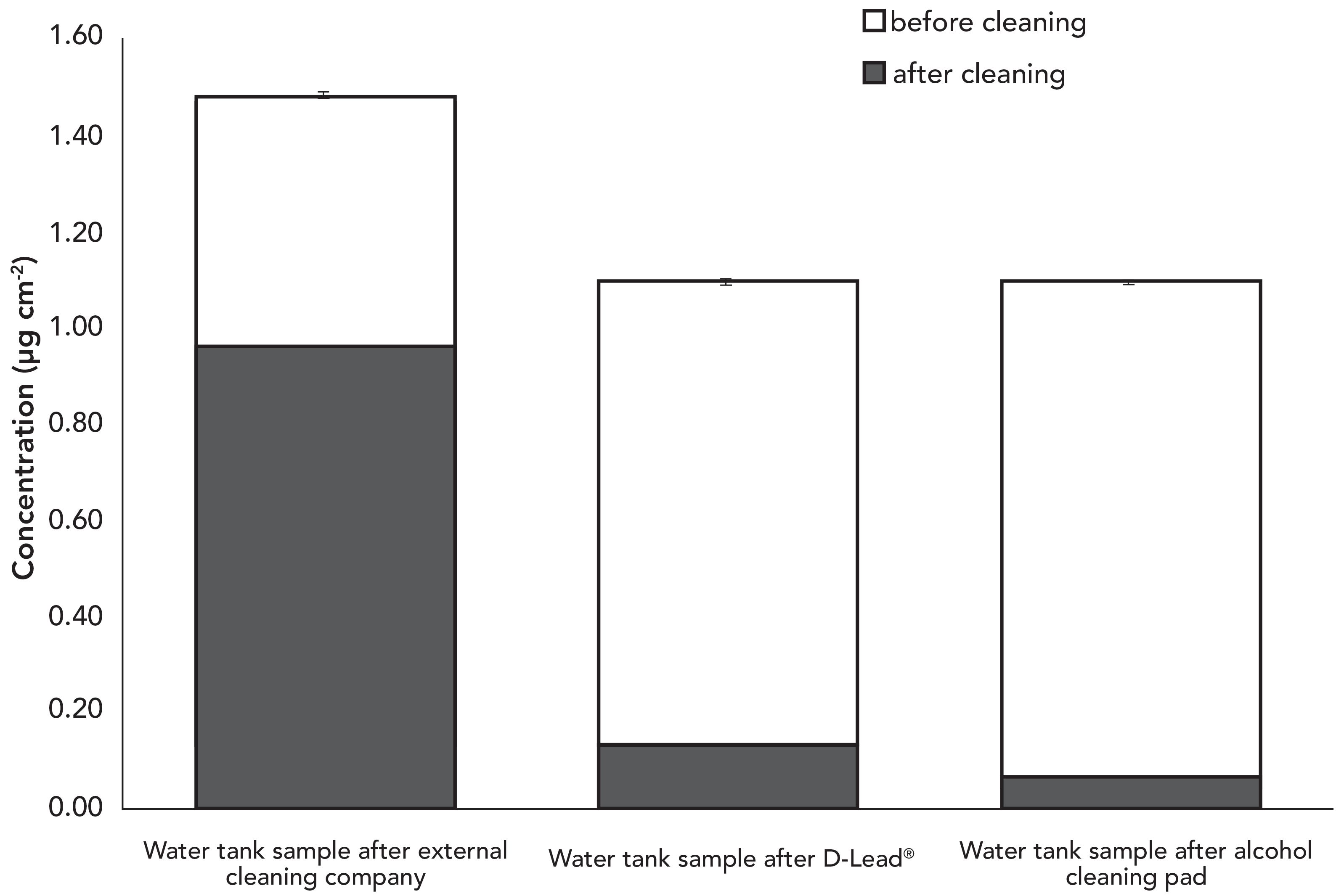
EGBE-containing decontaminants performed much better on a smooth surface. Figure 6 shows that wiping the stainless-steel surface of the top panel of the water tank reduced the lead concentration to 0.07 μg/cm2, or 31% below the EACO limit. Compared to the external cleaning company, which only removed 36% of lead on the water tank, an EGBE-laced decontaminant was able to remove 93% of lead on the surface.
Figure 6: Surface lead concentration on the top panel of the water tank bullet catch before and after cleaning by the external cleaning company, a commercial EGBE-containing lead decontamination product, and alcohol cleaning pad.
EGBE Decontaminants vs. Alcohol Cleaning Pads
To verify if the thoroughness of cleaning has a greater impact on lead abatement, another test used alcohol pads. The stainless-steel top surface of the water tank was wiped in another, closely-spaced area using alcohol pads (70% w/w isopropanol) and the extent of lead removal was similar to having used an EGBE decontaminant (Figure 6). This confirmed that the precise product used was less important than the cleaning technique itself, which is in agreement with the findings in a previous study (17).
Lead Concentration on Surfaces in NFLS-O Outside the Firearms Section
Other areas within the NFLS-O laboratory were selected as high-risk places for potential lead cross-contamination by secondary or tertiary transfer. Because they were not expected to have high endogenous levels of lead, the maximum acceptable threshold for comparison was lowered to the EACO residential limit of 0.043 µg/cm2 (14). Because GSR and explosives are analyzed in the trace evidence section, several different surfaces were tested. Several different surfaces were also selected in the biological evidence recovery section, where firearms can be examined. Biology examiners also share a photocopier with firearms examiners, which may be another point of secondary or tertiary lead transfer. Areas outside the firearms section, such as general entry doors and a display table, were tested, along with entrances to bathrooms used by firearms examiners.
Figure 7 shows that all lead concentrations in the areas outside the firearms section were below the EACO residential limit. A few notable areas included a convection oven in the trace evidence section (specifically the research and development [R&D] room that is separate from the operational trace room), an exhibit locker in the biological evidence recovery section that typically holds firearms for examination, the shared photocopier, and the firearms display table. The higher lead levels from the oven, exhibit locker, and display table are due to the nature of samples that come into contact with those surfaces.
Figure 7: Concentration of lead on surfaces outside the firearms work area. Some standard deviations were too small to be shown in the graph.
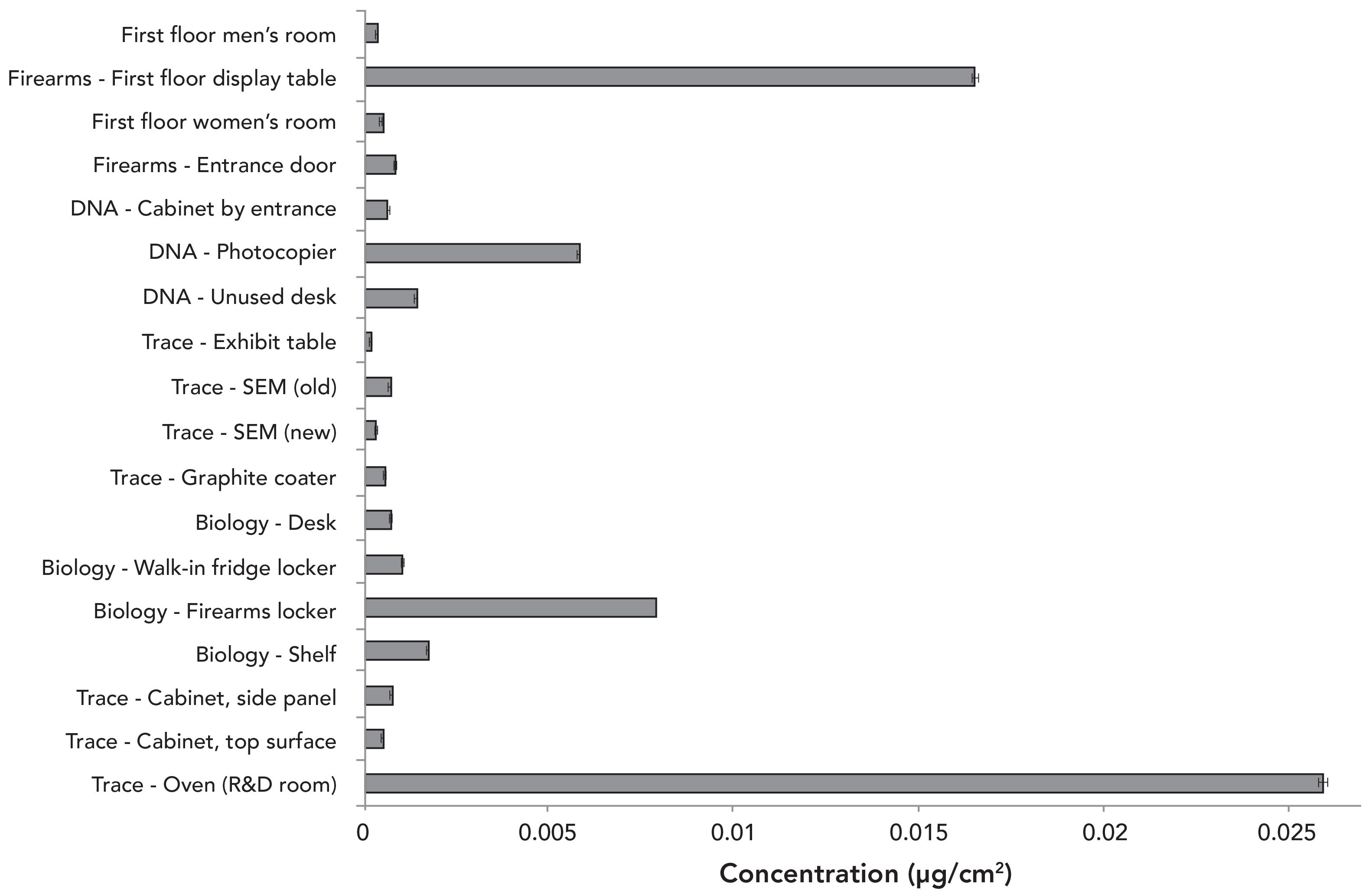
Although the current concentration on the surface of the photocopier is 14% below the EACO limit, it is much higher than the rest of the working area where it is found, indicating a single point of common contact with firearms examiners who cause cross-contamination.
Conclusions
A method for surface lead analysis using ICP-MS was validated using dust SRM 2584. Standard addition compensated for matrix effects whereas internal standardization with rhodium compensated for the nebulizer clogging by occasional particulate matter in sample solutions despite centrifugation, decantation, and dilution. Application of the method to real samples from a forensic firing range revealed problematic lead concentrations that were not reduced to the EACO limit by an external cleaning company, even on smooth surfaces. In contrast, using a consumer EGBE-containing lead decontamination product was effective in reducing lead concentrations on smooth surfaces below the EACO limit, as was using 70% w/w isopropanol pads.
On the other hand, no cleaning method tested in this study could reduce the lead concentration on the concrete floor in the firing range to an acceptable level. Only nonporous surfaces should be used in firing ranges to enable adequate cleaning. Given that unacceptable lead levels were found in a forensic laboratory firing range, even more problematic lead levels are expected in training and recreational firing ranges where the frequency of firearm discharge is significantly higher.
The lead surface concentration outside the firearms section of the facility was below the safe limit for residential living, indicating that secondary transfer was mitigated. Nonetheless, gloves should still be worn when touching surfaces potentially exposed to GSR, such as exhibit lockers for firearm storage, the oven in the trace laboratory, and the display table outside the firearms section, to avoid direct contact with lead-containing particles. The abnormal lead surface concentration on the photocopier shared with firearms examiners indicates a point of cross-contamination.
Future work will aim to simplify the ICP-MS method using external calibration with standard solutions containing 5% (v/v) HCl and 5% (v/v) HNO3 (instead of standard addition) along with internal standardization. Vacuuming will also be assessed for lead abatement on concrete floors in the firing range.
Acknowledgments
The authors gratefully acknowledge the financial support of the Natural Sciences and Engineering Research Council of Canada (Grant No. RGPIN-2018-04250) and of the Royal Canadian Mounted Police–National Forensic Laboratory Services (RCMP–NFLS). The authors thank Denis Leflèche of the RCMP–NFLS for providing the SEM images in this study. Lily Huang, one of this article’s authors, is grateful to Queen’s School of Graduate Studies for a graduate award.
References
(1) F. Saverio Romolo and P. Margot, Forensic Sci. Int. 119, 195–211 (2001).
(2) A. Martiny, A.P.C. Campos, M.S. Sader, and M.A.L. Pinto, Forensic Sci. Int. 177, E9–E17 (2008).
(3) O. Dalby, D. Butler, and J.W. Birkett, J. Forensic Sci. 55, 924–943 (2010).
(4) M.A.S. Laidlaw, G. Filippelli, H. Mielke, B. Gulson, and A.S. Ball, Environ. Health Global Access Sci. 16, 1–15 (2017).
(5) J.T. Walker, J. Crim. Law Criminol. 31, 497–521 (1940).
(6) J.S. Wallace, Chemical Analysis of Firearms, Ammunition, and Gunshot Residue (CRC Press, Boca Raton, 2008).
(7) G. Vanini, R.M. Souza, C.A. Destefani, B.B. Merlo, T.M. Piorotti, E.V.R. de Castro, M.T.W.D. Carneiro, and W. Romão, Microchem. J. 115, 106–112 (2014).
(8) Mayo Clinic, Lead Poisoning, 2016: https://www.mayoclinic.org/diseases-conditions/lead-poisoning/symptoms-causes/sync-205354717 (accessed May 2, 2018).
(9) D. Bellinger, A. Leviton, J. Sloman, M. Rabinowitz, H. L. Needleman, and C. Waternaux, Pediatrics 87, 219–227 (1991).
(10) K.N. Kim, H.J. Kwon, and Y.C. Hong, Neurotoxicology 53, 193–200 (2016).
(11) B.L. Gulson, K.J. Mizon, M.J. Korsch, J.M. Palmer, and J.B. Donnelly, Sci. Total Environ. 303, 79–104 (2003).
(12) B.L. Gulson, C.W. Jameson, K.R. Mahaffey, K.J. Mizon, N. Patison, A.J. Law, M.J. Korsch, and M.A. Salter, Environ. Health Perspect. 106, 667–674 (1998).
(13) CBC News, V.L. Nicholson, K. Kubinec, RCMP employee blows whistle on lead contamination risk from firing range, 2017: https://www.cbc.ca/news/canada/manitoba/i-team-rcmp-whistleblower-lead-risks-1.4371446 (accessed October 26, 2017).
(14) Environmental Abatement Council of Ontario, Lead Guideline For Construction, Renovation, Maintenance or Repair (EACO, Ontario, 2014), pp. 1–54.
(15) Midwest Research Institute, Analysis of Composite Wipe Samples for Lead Content: Final Report (U.S. Environmental Protection Agency, Washington, D.C., EPA Report 747-R-96-003, 1996).
(16) ESCA Tech Inc., Wipes - D-Lead® Surface Wipes for Lead Paint Dust Cleanup, 2014: https://www.esca-tech.com/ProductDetail.php?category=1100&productnum=PD (accessed January 30, 2018).
(17) D.Q. Rich, G.G. Rhoads, L.M. Yiin, J. Zhang, Z. Bai, J.L. Adgate, P.J. Ashley, and P.J. Lioy, Environ. Health Perspect. 110, 889 (2002).
(18) P.E. Rasmussen, C. Levesque, M. Chénier, and H.D. Gardner, Sci. Total Environ. 443, 520–529 (2013).
(19) B. Mravic, D. Mahulikar, G.N. Violette, E. Shapiro, and H. J. Halverson (Olin Corporation) US patent US5814759A, 1998.
(20) M. Jiang, Y. Nakamatsu, K.A. Jensen, and U. Satoshi, Atmos. Environ. 82, 364–374 (2014).
Lily Huang and Diane Beauchemin are with the Department of Chemistry at Queen’s University in Kingston, Ontario. Claude Dalpé is with the Royal Canadien Mounted Police at National Forensic Laboratory Services in Ottawa, Ontario. Direct correspondence to: diane.beauchemin@queensu.ca

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.