Feasibility Study of Portable X-ray Fluorescence (pXRF) for the Determination of Iodine in Pharmaceutical Products
Portable XRF has been found to be an efficient and economic analytical tool for elemental analysis in pharmaceutical products. This study explores the use of pXRF for determination of iodine in multivitamins.
A quick and accurate method for the determination of iodine in pharmaceutical products is critical for manufacturers to ensure product quality and to comply with the legal requirements as determined by authorities such as the Therapeutic Goods Administration (TGA) in Australia. As a noninvasive and nondestructive method of testing that requires little to no sample preparation, pXRF has been found to be one of the most efficient and economic analytical tools for elemental analysis in a wide range of applications. In this study, an approach using pXRF offers another option for the determination of iodine in multivitamin tablets, which is proving superiority for quality control on the production line.
Iodine is an essential trace element that plays an important role in human growth and development. Both iodine deficiency and iodine excess are detrimental to human health (1).
Iodine is often added in multivitamin tablet products, with concentrations ranging from 50 to 150 µg per tablet. Some special sea kelp tablets contain relatively high levels of iodine. Given that an elevated concentration of iodine is associated with various negative health effects, a quick and reliable method for the determination of iodine is necessary for the purposes of quality control and product release.
The conventional method to determine iodine for pharmaceutical manufacturers is highly time-consuming, due to the extraction process required. It has been evidenced that inductively coupled plasma–optical emission spectrometry (ICP-OES) and inductively coupled plasma–mass spectrometry (ICP-MS) are effective means of measuring iodine in pharmaceuticals (2). However, both methods require lengthy procedures for sample dissolution and extraction and the setup of the required instruments. In addition, neither method is practical as a quick on-site testing method for quality control.
This study explored the feasibility of iodine determination in pharmaceutical products using portable X-ray fluorescence (pXRF), which has been recognized as an efficient tool and as the first-line screening method of choice in many industries. This study aims to develop both a quick approach for homogeneity testing of mixed product powders, and an analytical method for the accurate determination of iodine (> 50 µg/g) in multivitamin tablets.
Materials and Methods
In this study, more than 10 multivitamin products from the local supermarket were collected. The products were manufactured with different compositions, with the iodine concentration ranging from 50 µg per tablet to 280 µg per tablet, according to their labels.
For accurate analysis of the tablets, they were blended into fine powders, and packed in an XRF sample cup sealed with Mylar TF-160-255 film. The analysis was performed on a portable XRF analyzer (SkyRay Explorer 7000). A user mode (or calibration) was developed based on the factory settings. This XRF analyzer provides an "expert" mode, in which the user can setup various instrument conditions including the excitation source, collimator, filters, peak signal calculations, and interference corrections. A test stand was used to ensure the sample test position is consistent. All samples were analyzed with the settings of 45kV 50 µA and 180 s. It was observed that after 120 s, the signals were almost consistent.
For the homogeneity tests, tablets may be determined directly, but care must be taken to ensure the tablets are placed in the same test position in front of the test window.
Results and Discussions
In this section, the method was assessed and discussed according to the ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2(R1) (3).
Specificity
Specificity is the ability to assess unequivocally the analyte in the presence of components that may be expected to be present. The most powerful and principal energy signal of iodine Ka is found at 28.612 keV, at which the possible spectral overlap may be from tin Kβ at 28.485keV, antimony at Kβ 29.73keV, and tellurium at Ka 27.47keV. In this study, a pXRF analyzer demonstrated a resolution of approximately 0.15 keV. No evidence of possible overlap from these interferences was observed, thus demonstrating the specificity of the method.
Accuracy
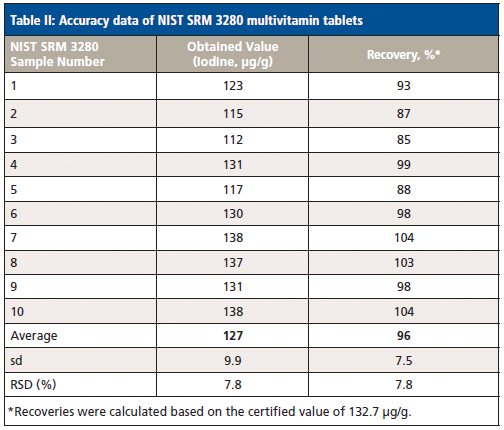
The label values of each product and the expected values of the spiked samples were used as the acceptable values to benchmark the measured results (Figure 1). In addition, NIST SRM 3280 multivitamin tablets were analyzed, and the certified value of iodine was used as the standard value for assessing the accuracy of the method. The initial calibration curve was established using pure iodine standards made from the mixture of potassium iodide and silicon dioxide. By analyzing all different multivitamin samples, including the NIST SRM 3280, an adjustment factor was input into the calibration curve to correct for matrix effects. Then, the adjusted calibration curve was verified by analyzing the NIST SRM 3280. Once consistent results were achieved, a generic calibration for the multivitamin matrix for all products was generated.
The accuracy test results are listed in Tables I and II.
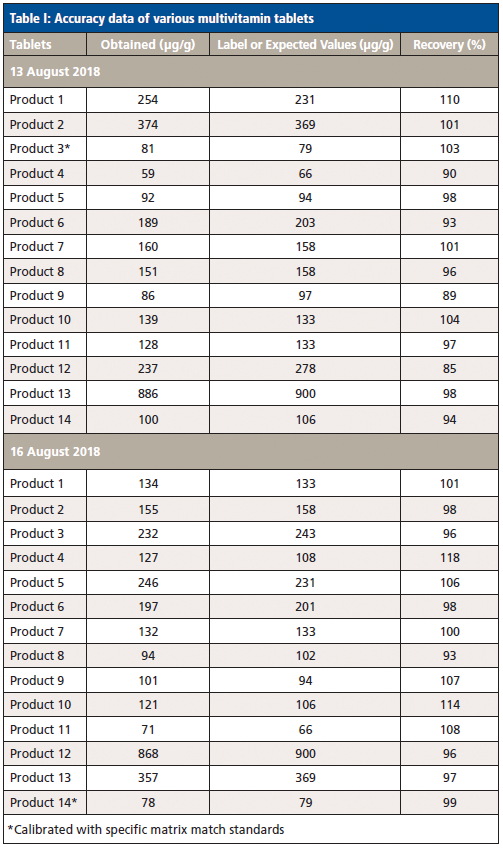
As a matter of fact, each tablet product has a different matrix. To obtain more accurate results for each individual product, the calibration curves may be fine-tuned for each specific tablet type.
Precision
In this study, short-term repeatability checks were carried out and the relative standard deviation (RSD%) in percentage was used to assess precision. Like any other instrumental method, the precision varies with the analyte concentrations. The RSD% is usually higher when the concentration is close to the reported limits. The precision test results at the level of around 80 µg/g and 100 µg/g are illustrated in Figure 2 and Figure 3.
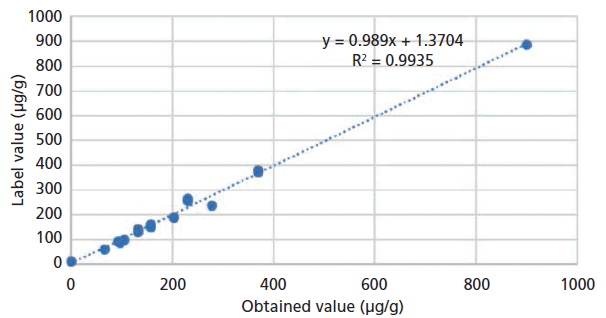
Figure 1: Correlation between the label values and the obtained values by the pXRF method.
The same precision test at the 80 µ/g level was performed on three different dates, and the RSDs (as %) were 7.8%, 9.5%, and 8.7%, respectively.
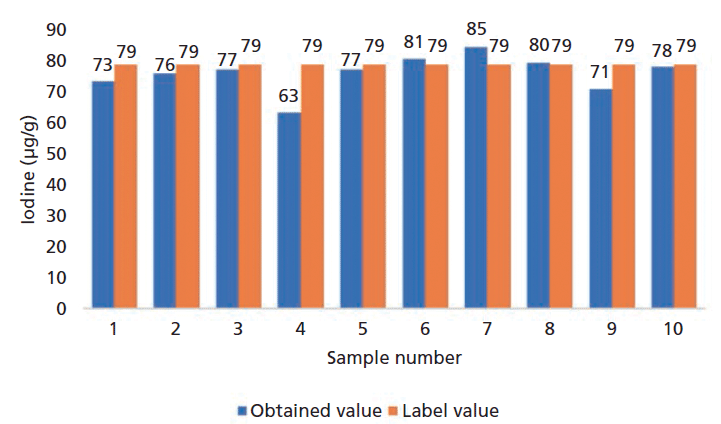
Figure 2: Iodine accuracy and precision test results at the level of ~ 80 µg/g. The average concentration of the iodine was 76 µg/kg with RSD = 7.8% (n = 10).
The precision achieved was comparable to the precision of other methods. The National Institute of Standards and Technology (NIST) reported precisions of 7.6–8.6% for iodine (~132 µg/g) on multivitamin certified reference material using the methods, such as ICP-MS and instrumental neutron activation analysis (INAA) (4).
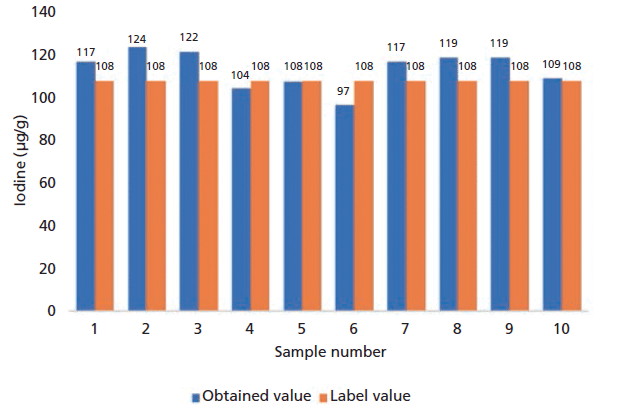
Figure 3: Iodine accuracy and precision test results at the level of ~ 100 µg/g. The average value of iodine in 10 tested tables was 113 µg/kg and the RSD% was 7.6%.
For the iodine content level at 500 µg/g, the RSD% valves were found consistently to be from 3 to 5%.
Detection Limit
In this study, the detection limit was reported as three times the standard deviation of a real multivitamin sample, with very low iodine concentration analyzed seven times. The typical detection limit of this method is around 20–30 µg/g.
Increasing the measurement time will improve the detection limit. The current measurement time was set as 180 s, further increasing the measurement time to 240–300 s will improve the detection limits to around 10–20 µg/g.
Linearity
The typical linearity of this method was up to 1000 µg/g with a coefficient of determination (R2) of greater than 0.999 (Figure 4), which covers the iodine concentration in almost any multivitamin product.
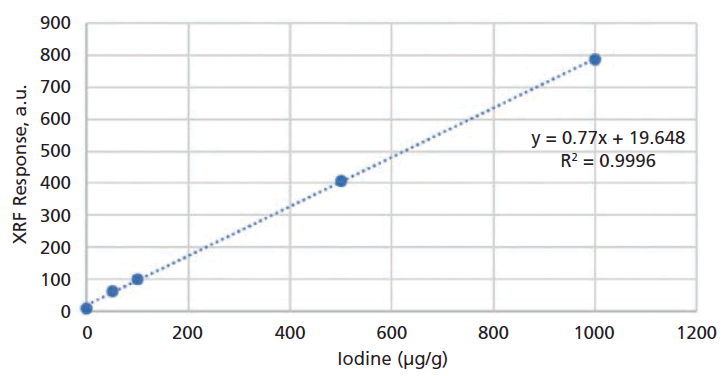
Figure 4: Iodine calibration curve.
It must be noted that, for an XRF method, the calibration curve established from the pure analyte may not be suitable for the real samples. Ideally, a calibration curve should be specifically established for each product using standard addition calibrations for each product to get the best results.
Range
The range of an analytical procedure is the interval between the upper and lower concentration (amounts) of analyte in the sample (including these concentrations) for which it has been demonstrated that the analytical procedure has a suitable level of precision, accuracy, and linearity.
With the tablet products sourced from the supermarket, the lowest concentration is 50 µg per tablet and the highest concentration is 280 µg per tablet; considering the tablet weight, the lowest concentration is 62.5 µg/g and the highest concentration is 900 µg/g. An RSD of approximately 15% at the low concentration of 62.5 µg/g, and an RSD of around 5% at the high concentration of above 300 µg/g, were observed.
Robustness
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters and provides an indication of its reliability during normal usage. In this study, the following variations were deliberately introduced, and the effects of these were assessed:
Effects of the Tablet Shape, Coating, and Size
The tablet may be placed in front of the XRF window for direct iodine measurement. However, the tablet position, size, shape, and coating will affect the test results. Direct measurement may be used for comparisons between each single tablet of the same product, each time the tablet must be placed in front of the XRF measuring window in the same manner.
For quantitative analysis, it is suggested to blend the tablets into powder before measurement, so that the effects of the tablet sizes and shapes are minimized.
Effects of Powder Density and Sample Depth in the XRF Cup
Once a fine powder was obtained, the powder was packed into an XRF cup by repeated tapping. The cup should be filled to at least 1.5 cm in depth. No significant difference in analytical signal was observed when the cup was filled to more than 1.5 cm. It was observed that the XRF signal intensity changed when the sample density changed.
Effects of Measurement Time
The longer measuring time will improve the measurement precision and the detection limit and quantity limits. However, when the measuring time is longer than 180 s, less improvement was observed.
Matrix Effects
Physical matrix effects result from the variation in the physical and chemical characteristics of the samples, which include sample composition, particle size, uniformity, homogeneity, and surface conditions. It was observed that the iodine signal was suppressed in some product matrices, but was not changed in other products. In general, pXRF is a technique that requires "full match" of the calibration standards and the samples in terms of the composition and test conditions if accurate results are required.
Applications
With the method developed, it takes about 10 min to complete the analysis, including blending tablets into powder, packing the sample powder into an XRF cup, and measuring in the pXRF analyzer.
With the feature of nondestructive and in-situ testing, pXRF is an ideal option for on-line monitoring of product quality at different stages of production.
In addition, the multivitamin tablets can be directly placed in front of the pXRF test window for iodine determination, which makes it an easy way to identify the difference or the homogeneity between tablets for the same type of product.
Conclusions
The analytical performance of the method for the determination of iodine in pharmaceutical products, such as multivitamin tablets, was evaluated in terms of accuracy, precision, and other parameters. The method presents advantages such as direct determination of iodine with minimum sample preparation and fast results within minutes, making pXRF an ideal tool for quality control purposes in the pharmaceutical production line. This method may potentially be applied for other elements such as iron, zinc, and selenium in multivitamin tablets and other products.
Acknowledgments
The author acknowledges Dr. Andrew Evans, Ms. Luminita Antin, Mr. Andrew Taylor and Ms. Xiao Teng Zhou for discussions of technical questions.
References
(1) Iodine, Fact Sheet for Health Professionals, Office of Dietary Supplements, National Institutes of Health, updated on 2nd March 2018.
(2) C. P. Shelor and P.K. Dasgupta, Analyt. Chimi. Acta 702, 16– 36 (2011).
(3) ICH Harmonized tripartite guideline, validation of analytical procedures; Text and Methodology Q2(R1), Current Step 4 version Parent Guideline dated October 27, 1994 (Complementary Guideline on Methodology dated November 6 1996, incorporated in November 2005).
(4) G. Turk, K. Sharpless, D. Cleveland, C. Jongsma, E. Mackey, A. Marlow, R. Oflaz, R. Paul, J. Siebler, R. Thompson, L Wood. L. Yu, R. Zeisler, S. Wise, J. Yen, S. Christopher, R. Day, S. Long, E. Greene, J. Harnly, I-P Ho, and J. Betz, J. AOAC Int. 96(6), 1281 (2013).
Michael Wu is with UBO Services Australia Pty Ltd. in Cherrybrook, Australia. Direct correspondence to: michael.wu@uboservices.com.au

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.
Smarter Sensors, Cleaner Earth Using AI and IoT for Pollution Monitoring
April 22nd 2025A global research team has detailed how smart sensors, artificial intelligence (AI), machine learning, and Internet of Things (IoT) technologies are transforming the detection and management of environmental pollutants. Their comprehensive review highlights how spectroscopy and sensor networks are now key tools in real-time pollution tracking.