Gas Analysis with the Nicolet iS50 FT-IR Spectrometer
Special Issues
Combining a high precision FT-IR spectrometer with a long pathlength gas cell provides a powerful tool for analyzing trace levels of contaminants in air and other gas mixtures.
Combining a high precision FT-IR spectrometer with a long pathlength gas cell provides a powerful tool for analyzing trace levels of contaminants in air and other gas mixtures (Figure 1). Two important applications of this are ensuring air quality and the purity of breathing oxygen and compressed air.

Figure 1: Rugged 10-m multi-pass white cell mounted in the Thermo Scientific Nicolet iS™ 50 spectrometer provides sub ppm detection for many pollutants.
QA/QC and Air Purity
Infrared gas analysis is critical in screening for trace contamination in aviator’s breathing oxygen (ABO). Both the U.S. Air Force and Navy have systems to verify that no dangerous contaminants are present.
Spectra were acquired in a 10-m gas cell from gas standards used to calibrate the Air Force method. Spectra were acquired with a 1 min scan time at 1 cm-1 resolution. The Thermo Scientific™ TQ Analyst™ software method was calibrated with spectra obtained from gas standards purchased from Scott Specialties Gases® and tested on a spectrum of the Navy test gas as shown in Figure 2.
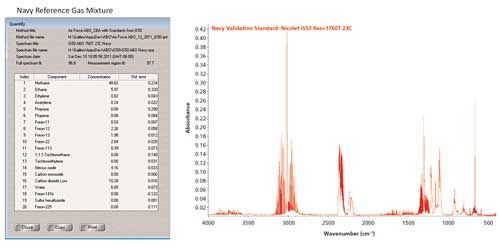
Figure 2: Calculated concentrations from a sample of the Navy reference sample containing trace levels of potential contaminants in aviator’s breathing oxygen.
These results (in ppm) show a standard error of less than 100 ppb for most of the 20 components. Similar methods are used in many industries to detect trace contamination and moisture.
Environmental and Air Monitoring
Volatile organic species and other pollutants are regularly detected using IR spectroscopy. These hazardous compounds may originate from a manufacturing process, land fill off-gas, vehicle exhaust, or a chemical spill. A major challenge in measuring pollutants in the air is the strong infrared absorbance for both water and CO2, which block many useful spectral regions. In many applications, continuous extractive sampling, Summa canisters, or Tedlar® bags are used to collect samples that can be pulled into the 10-m gas cell. In high humidity situations, the gas cell may be heated to ensure water does not condense.
A spectral resolution of 0.5 cm-1 may also be necessary to create analysis windows between the water and CO2 peaks, as shown in Figure 3.
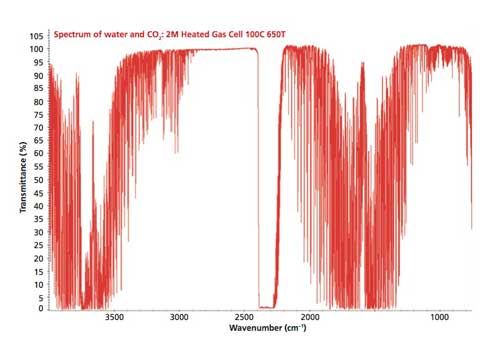
Figure 3: Spectrum showing very strong features corresponding to water and CO2 that make air quality and combustion monitoring a challenge.
Conclusion
We have presented an overview of gas analysis application areas where infrared spectroscopy has proven valuable. The Nicolet iS50 FT-IR spectrometer is designed with flexibility to analyze a variety of sample types with ease. The combination of high sensitivity and a full suite of software features, specifically designed for gas analysis, creates a world-class solution to a broad range of applications.©2015 ©Thermo Fisher Scientific Inc. All rights reserved. Scott Gas is a part of Air Liquide America Specialty Gases LLC. Tedlar is a registered trademark of E. I. du Pont de Nemours and Company. All other trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. Specifications, terms and pricing are subject to change. Not all products are available in all countries.

Thermo Fisher Scientific
5225 Verona Road, Madison, WI 53711
tel. (800) 532-4752, (608) 276-6100; fax: (608)273-5046
Website: www.thermofisher.com/ftir

Best of the Week: AI and IoT for Pollution Monitoring, High Speed Laser MS
April 25th 2025Top articles published this week include a preview of our upcoming content series for National Space Day, a news story about air quality monitoring, and an announcement from Metrohm about their new Midwest office.
LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.