Happy Sesquicentennial, Spectroscopy!
David Ball celebrates the 150th birthday of spectroscopy.

This year, 2010, is the 150th anniversary of the formal appearance of spectroscopy (the technique, not this publication). Happy 150th birthday, spectroscopy!

David W. Ball
Birth of Spectroscopy?
In 1860, physicist Gustav Kirchhoff and chemist Robert Bunsen (1) published a long article detailing their investigations with a spectroscope (Figure 1). In the article, they proposed that the lines of light in the spectrum, which had been noted for years, came from the elements in the sample that was exposed to a flame source. Additionally, Kirchhoff and Bunsen pointed out lines in the spectrum that, upon comparison to other spectra, were not present and proposed that these lines of light came from a heretofore unknown element. This element was named cesium (or caesium, as it is spelled outside the U.S.), so named after the blue lines in the spectrum (caesius, L., "sky blue"). The demonstration of the chemical basis of spectral lines was a watershed in the development of modern science, and the new tool sparked investigations that eventually led to the development of quantum mechanics and other aspects of modern science.

Figure 1: The diagram of the spectroscope published by Kirchhoff and Bunsen in their ground-breaking 1860 article that established the chemical basis of spectroscopy.
As mentioned, observing the spectrum of a light source, and lines of bright or dark lines superimposed on the spectrum, was not new. Indeed, Kirchhoff and Bunsen did not actually invent the spectroscope, as is widely surmised (even by this author). The spectroscope actually was invented by Joseph von Fraunhofer, a German optician. Although spectra had been generated since Isaac Newton in the 1670s using a prism, Fraunhofer was apparently the first to construct a diffraction grating and incorporate it into the spectroscope (2). Fraunhofer's gratings were good enough that he could use his spectroscope quantitatively for the first time. In particular, Fraunhofer pointed his spectroscope at the sun and recorded the positions of hundreds of dark lines superimposed on the broad spectrum. Dark lines on the solar spectrum had been noted previously by English chemist William Wollaston in 1802, but his work was only qualitative. Fraunhofer rediscovered them in 1812, and was eventually able to pinpoint the positions of over 500 dark lines, now called the Fraunhofer lines. The most prominent lines were labeled by the capital letters A through K, and less prominent lines with lowercase letters and numbers. Figure 2 shows some of the more prominent Fraunhofer lines in the solar spectrum.
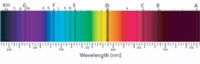
Figure 2: Some of the Fraunhofer lines in the solar spectrum.
What Kirchhoff and Bunsen did was explain where these dark lines came from in terms of chemical composition, ultimately, the elements. By using a spectroscope to generate spectra from a wide variety of samples, they were able to deduce that the lines of light in a spectrum were related to the elements that were present in the sample. Thus, they established spectroscopy as an analytical tool rather than just a technique for generating a rainbow. The field has blossomed a bit since.
Given the progressive nature of science, it might be improper to point to 1860 as the year that spectroscopy was "born." Both Kirchhoff and Bunsen, separately and together, made advances before this that certainly contributed to establishing spectroscopy as a legitimate field of scientific inquiry. However, it was the prediction of a new element (and its subsequent demonstration as correct) that firmly established spectroscopy as something more than a visual curiosity. So, let's take it as the birth year of spectroscopy and reflect on 150 years of the study of matter with light.
Gustav Kirchhoff
Gustaf Robert Kirchhoff (Figure 3) was born in what was then East Prussia (now an enclave of Russia) in March 1824. An intelligent youth, he entered the Albertus University of Königsberg in 1843, graduating in 1847. However, as early as 1845, Kirchhoff first enunciated his laws of electrical circuits, building on work by Georg Ohm and anticipating James Clerk Maxwell's work on electromagnetism. These two simple statements are essentially conservation laws and are still taught to electrical students today. I will paraphrase them as follows:
- The current flowing into a node of an electrical circuit equals the current flowing out of that node, and
- The sum of the electrical voltage in any closed circuit is zero.

Figure 3: Gustav Kirchhoff.
After graduating, Kirchhoff worked in Berlin and Breslau before moving to the University of Heidelberg in the mid-1850s, where he met Robert Bunsen and began a very fruitful collaboration. In the 1860s, Kirchhoff coined the term "blackbody radiation" and made significant contributions to its study, which was problematic and ultimately led to the development of quantum theory by Planck in 1900 (indeed, Planck was a student of his at Berlin and was appointed to the university's faculty after the latter's death). Studies with spectroscopes in the late 1850s led to Kirchhoff proposing that the emission spectrum and absorption spectrum of a substance were the same, a crucial concept in spectroscopy. His seminal studies in spectroscopy with Bunsen came in between 1859 and 1861, when he and Bunsen identified elements in the sun from its solar spectrum. He also made contributions to the thermodynamics of phase changes and the optics of refraction. In 1875, Kirchhoff became a professor of mathematical physics in Berlin, when failing health made experimental work more onerous.
Kirchhoff died in 1887 and is buried in Berlin, where his grave is still visible (Figure 4) (3).

Figure 4: Kirchhoff's grave in Berlin.
Robert Bunsen
Robert Wilhelm Eberhard Bunsen (Figure 5) was born in Gottingen, Germany, in March 1811. At the young age of 20, he received his Ph.D. in chemistry from the University of Gottingen, where he became a lecturer before moving to the Polytechnic School of Kassel and then to the University of Marburg. There, he specialized in studies of tetramethyldiarsine (called cacodyl) and its derivatives. These studies were extremely dangerous because cacodyl burns spontaneously in air; indeed, Bunsen suffered from slight arsenic poisoning, and lost his right eye due to a cacodyl explosion. In time, his studies of arsenic chemistry brought him his initial scientific fame. In 1852, Bunsen moved to the University of Heidelberg, where he met Kirchhoff. In 1855, Bunsen and his mechanic colleague Peter Desaga redesigned a gas burner so that it burned cleaner and hotter than previous models; although the Desaga family had the exclusive right to market these new burners for years, they are known as Bunsen burners to generations of science students. (Bunsen apparently had a rather modest personality and refused to patent the new burner.)

Figure 5: Robert Bunsen.
The Bunsen burner was crucial to Kirchhoff's and Bunsen's spectral studies because it allowed samples to be heated hotter and cleaner, so the spectra measured by the researchers was demonstrably due to the sample rather than the flame itself.

Figure 6: Bunsen's grave in Heidelberg.
Bunsen not only was rather modest, but he was a consummate professional, being very dedicated to his educational responsibilities. The list of students who worked under him is impressive given the contributions to science that they ended up making: Mendeleev, Lothar Mayer, Tyndall, Beilstein, Lenard. The influence Bunsen had over the development of modern science lasted long after his retirement at age 78. After he retired, Bunsen turned his interest to geology, a life-long interest. Bunsen died in August 1899, aged 88, right before the birth of modern science, and is buried in Heidelberg (Figure 6).
Since 1990, PerkinElmer and theGerman Working Group for Applied Spectroscopy have awarded the Bunsen–Kirchhoff Award for outstanding achievements in analytical spectroscopy. The award is, of course, named in honor of the field's founders.
David W. Ball is a professor of chemistry at Cleveland State University in Ohio. Many of his "Baseline" columns have been reprinted in book form by SPIE Press as The Basics of Spectroscopy, available through the SPIE Web Bookstore at www.spie.org. His book Field Guide to Spectroscopy was published in May 2006 and is available from SPIE Press. He can be reached at d.ball@csuohio.edu; website: academic.csuohio.edu/ball.
References
(1) G. Kirchhoff and R. Bunsen, Annalen Phys. Chem. 110, 161–89 (1860).
(2) J. Fraunhofer, Annalen. Phys. 74, 337–78 (1824).
(3) A.S. Everest, Phys. Educ.4, 341–343 (1969).
















