Illicit Drug Analysis in Blood Samples with Multivariate Analysis Using Surface-Enhanced Raman Spectroscopy
This study aims to discriminate different types of illicit drugs (MDMA and THC) in blood samples using surface-enhanced Raman spectroscopy (SERS) combined with chemometric techniques including principal components analysis (PCA) and partial least squares discriminant analysis (PLS-DA). A PLS-DA classification model was built using a training data set containing Raman spectra from control and experimental groups (drug-detected blood). PLS-DA was performed for discrimination and classification among blood samples. The scores obtained in the PLS-DA model were used to evaluate the performance of the created model. The leave one out cross-validation (LOOCV) method was used for calibration and validation of the PLS-DA model. In the study, it was observed that the SERS method and chemometric techniques together could be used in drug analysis, even at low concentrations in complex body fluids such as blood. As a result, Raman spectroscopy with PCA and PLS-DA methods of data analysis could be used extensively to build similar or different classification models.
Illicit drug use or addiction has become an important problem all over the world, as it causes many serious problems (1). The World Drug Report in 2020 stated that the estimated number of past-year users of any drug utilization globally increased from 210 million to 269 million from 2009–2018. Around 20.5 million people globally were estimated to have used “ecstasy” in the past year, corresponding to 0.4% of the global population aged 15 and older in 2018 (2).
Since 1971, amphetamine, meth- amphetamine, methcathinone and “ecstasy”-group substances (3,4-methylenedioxymethamphetamine [MDMA] and its analogues) have been called amphetamine-type substances (3). Ecstasy and cannabis (tetrahydrocannabinol [THC]) are two of the most frequently encountered drugs in blood analyses for the detection of drug use. THC is the most important psychoactive ingredient in cannabis (4).
Biological samples such as blood, urine, hair, nails, and saliva are used to determine whether a person uses drugs or not (5). Today, different analysis methods are used for body fluid identification. These analysis methods can be classified as chemical tests, immunological tests, protein catalytic activity tests, or spectroscopic and microscopic methods. The methods are applied for the harmless examination of obtained evidences, such as blood, urine, and saliva, as they continue to develop. Although the results of both screening and validation tests are reliable, the methods take time and may delay treatment (6,7).
Raman spectroscopy is one of the most recommended methods, since it is a method that does not damage the sample and provides rapid results, with even a small amount of sample being sufficient for examination (7–11). Raman spectroscopy has been increasingly used in all scientific areas in recent years in different analyses, and has enabled the determination of illicit drugs without using additional chemicals (8). However, although Raman spectroscopy has many advantages, it is sometimes not sufficient by itself to analyze a small amount of a component in blood. This is because the components in biological samples may generate very weak Raman peaks. For this reason, the use of the surface-enhanced Raman spectroscopy (SERS) technique is more advantageous in Raman examinations. The SERS method significantly increases the applicability of Raman spectroscopy (12). The SERS method is a spectroscopic technique used to induce vibrational transmission of adsorbed molecules on a rough metallic surface. Thanks to their electromagnetic and charge transfer mechanisms, metallic nanoparticles (Ag, Au, and others) are widely used in SERS applications. In addition, being able to control properties such as the size and the shape of nanoparticles facilitated the widespread application of the SERS technique in biomedical analytics and clinical diagnostics (13–16).
It is difficult to obtain an exact result, due to many different reasons (such as the classification of the spectra obtained by Raman spectroscopy examination of biological samples, as well as the limited number of samples). For this reason, chemometric methods are needed to classify many variables (17) and used for both qualitative and quantitative analysis of the data while being categorized as supervised or unsupervised methods. Principal component analysis (PCA) is an unsupervised method, and is convenient for the analysis of unknown spectra. A small amount of unknown data can be obtained, such as principal components (PC) and loadings (eigenvectors). Another preferred reason for the application of PCA to the data obtained from the spectra in the studies is that only some components in a mixture are known (18). Partial least square (PLS) regression is a supervised method, and generally applied in cases where there is a limited number of samples. Calibration and validation are a two-stage process for the supervised methods (19–21). Conditions such as the fact that the PCA method is an unsupervised method do not necessarily reveal the relevant patterns, and the performance of PLS largely depends on the number of components to be used in discriminant analysis (DA) (17). PLS-DA is a linear classification method based on PLS regression, and it is the most popular chemometric tool used for qualitative and quantitative modeling of multidimensional data. The large data in human blood samples can give rise to problems in the analysis. Thus, the study aims to discriminate different type of illicit drugs (MDMA and THC) in blood samples using the SERS method combined with multivariate analyses. For this purpose, SERS spectra of the blood samples were analyzed. PLS-DA and PCA were performed for discrimination and classification among blood samples.
Materials and Methods
Preparation of Samples
This study was approved by the Health Sciences Central Ethical Committee (14.10.2021/01). Consent forms were signed and a total of 22 blood samples were taken from two groups: an experimental group (drug users) who were brought to the health service for different reasons, and the control group (non-drug users) in whose blood drugs were not contained. Blood samples were taken into ethylenediaminetetraacetic acid (EDTA) tubes and stored at −20 oC in a freezer until the examination was made. In all examinations, slides that were compatible with Raman spectroscopy and did not change the peak of the sample were used. Silver nanoparticles (AgNPs) were synthesized by reduction of AgNO3 using trisodium citrate according to the Lee and Meisel method (22). Chemicals were supplied to synthesized AgNPs from Sigma Aldrich. An ultraviolet-visible (UV-Vis) spectrophotometer and Scanning Electron Microscopy (SEM-EDS) analysis unit (JEOL 5500-OXFORD Inca-X) were used to characterize AgNPs. Before SERS measurements, 15 μl of AgNPs and 15 μl of blood samples were separately dropped on the slides and allowed to dry at room temperature and in the dark. After drying, Raman spectra of blood samples were obtained by the SERS method.
Equipment
All samples were measured with Renishaw’s Via Raman spectroscopy instrument equipped with a charge-coupled device (CCD) and the same parameters. The parameters were taken as 5 mW laser power, 50x objective, 785 nm diode laser for excitation, and 40 s exposure time. Raman bandwidth was chosen as 100 to 3200 cm-1.
Data Analysis
Spectral collection and data pre-processing (such as smoothing, fourth polynomial baseline correction, and vector normalization) from Raman spectroscopy were acquired using the WiRE 3.2 software (Renishaw plc). In the study, each sample was analyzed five times, and then averaged to form a spectrum representing one sample. A total of 889 spectral data were created in the region between 500–1600 cm-1, which forms the fingerprint region and has different peaks, from the Raman spectra of 22 (11 positive, 11 negative) blood samples. The data were transferred to the MATLAB (Matlab 7.13, The Mathworks) program and analyzed using PLS Toolbox (Eigenvector Research) for Matlab users, accounting for both the PCA and PLS-DA methods.
The PCA method was used for the analysis of 11 blood samples (2 MDMA, 1 THC + MDMA, and 8 THC), which were determined to be different drugs. Second, the PLS-DA method was used to optimize the separation between drug-containing and drug-free blood.
Results and Discussion
SERS Analysis
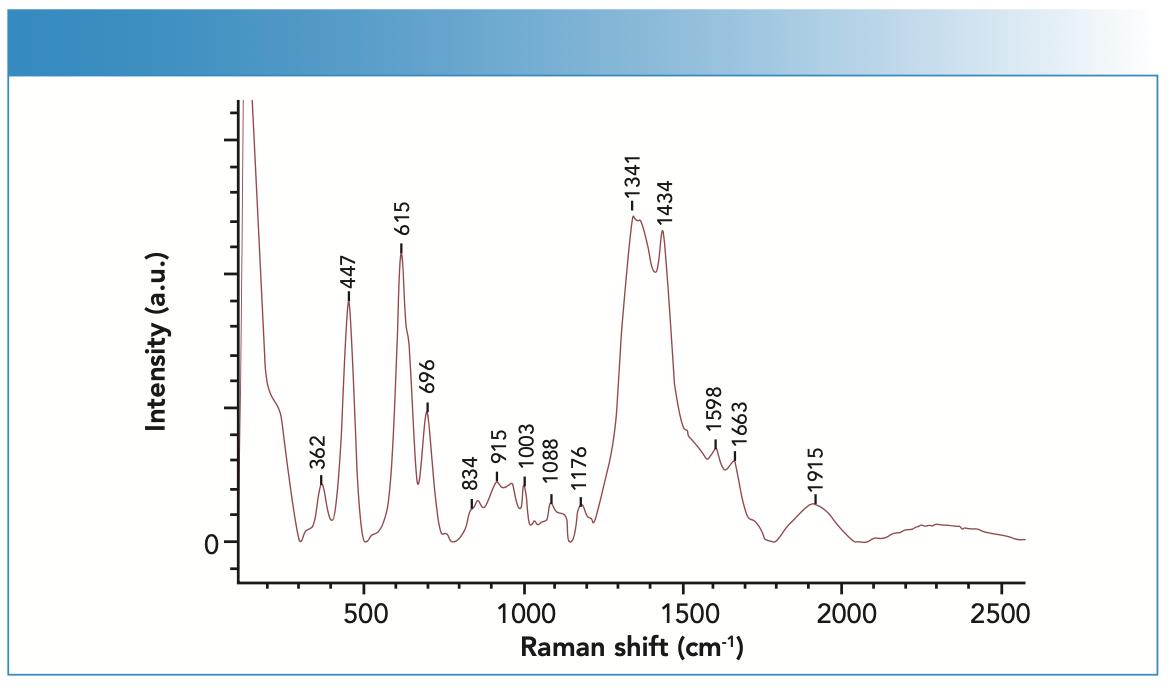
In the study, a SERS spectrum of the blood sample was obtained, and Raman bands were analyzed (Figure 1). The Raman band at 495 cm-1 involved vibrational modes of amino acids (23). The peaks at 1003, 1341, 1434, 1598 and 1663 cm-1 were assigned to hemoglobin and its derivatives (10). Raman bands at 1341 and 1434 cm-1 corresponded to deformations of the carbon rings and the characteristic bending motions of the CH, CH2, and CH3 groups amino acid deformation modes. The bands at 1003 cm-1 and 1663 cm-1 involved stretching motions of the phenylalanine, C-C skeletal and amide, C=C groups in unsaturated fatty acids correspondingly (24,25). The bands at the 1176 cm-1 and 1598 cm-1 correspond to the vibrations of C-C and C=C stretching.
FIGURE 1: SERS spectrum of the blood sample.
The SERS spectra obtained in blood samples containing THC and MDMA are shown in Figure 2 and Figure 3, respectively. It was observed that the strong SERS bands obtained in blood samples containing THC were the same in the fingerprint range of 500–1600 cm-1 (Figure 2). However, it was observed that there were changes at the 615, 696, 1341, and 1434 cm-1 bands of blood samples containing different amounts of THC. The weak Raman bands at 830–860 cm-1 were assigned to stretching of the middle tetrahydropyran ring, the weak Raman bands at 1003 and 1088 cm-1 corresponded to phenyl ring peak intensities, and the weak Raman bands at 1341 cm-1 were assigned to CH deformation modes (26). The characteristic Raman bands of blood samples containing different amounts of THC were detected at 712, 1006, 1310, 1390, 1450, and 1590 cm-1. These bands corresponded to vibrations of, respectively, (C-H) deformation, (C-C) stretching, (C-H) deformation, (=C-H) deformation, (C-H) deformation, and C=C stretching (27).
FIGURE 2: SERS spectra of the blood samples from patients with THC content.
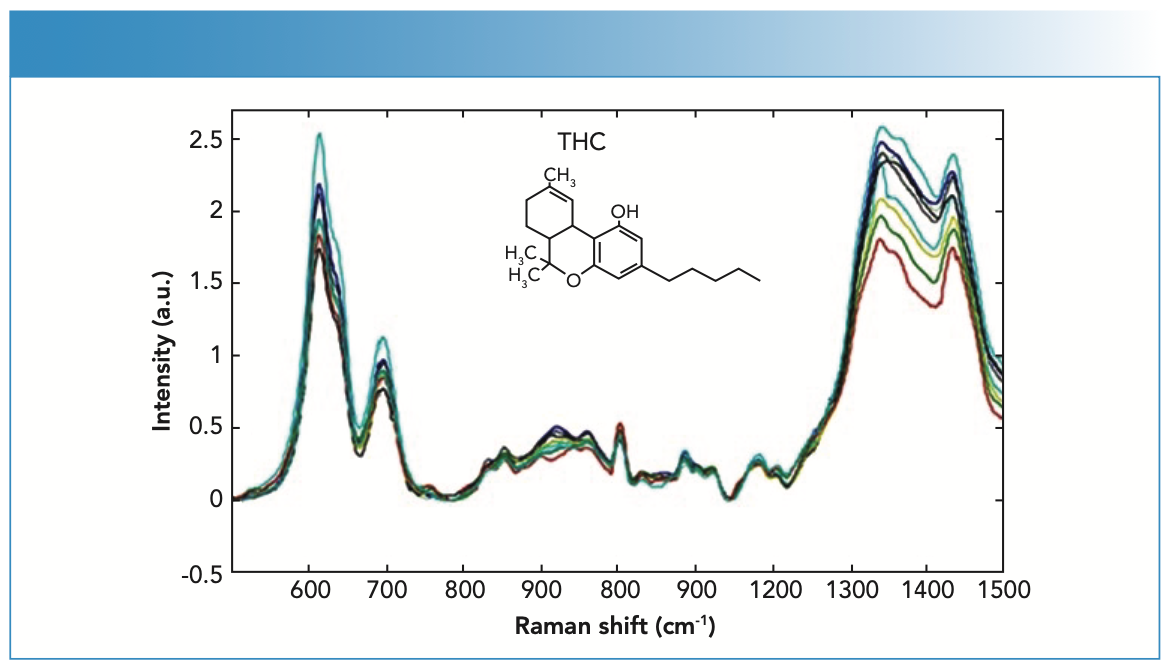
FIGURE 3: SERS spectrum of the blood serum samples from a patient with MDMA content.
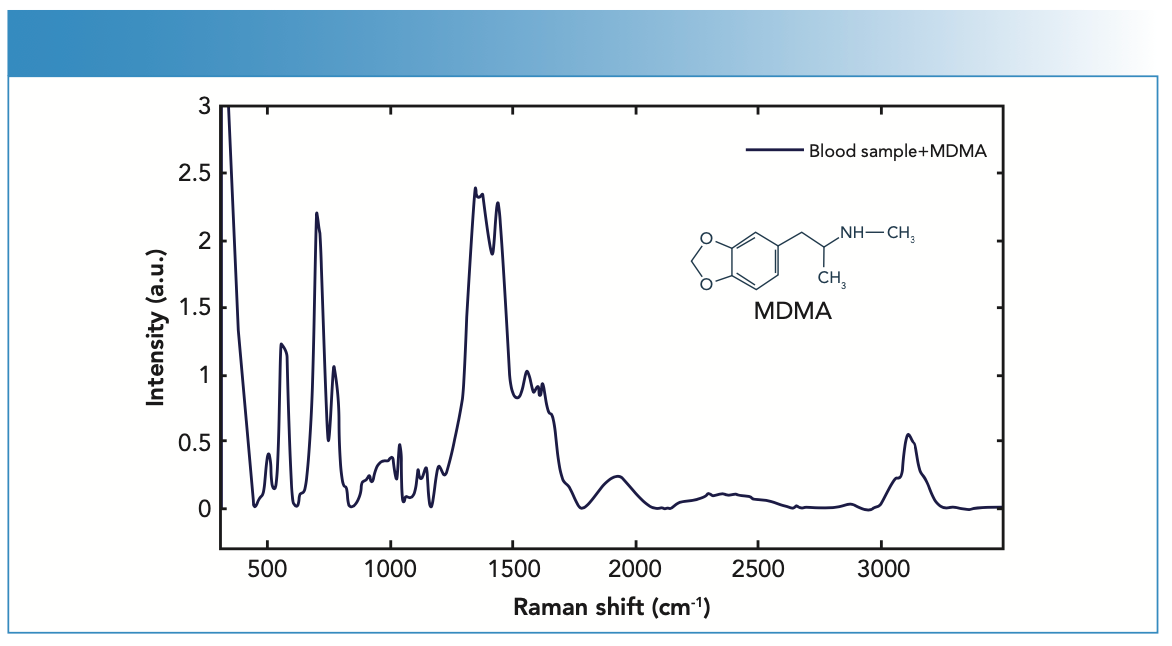
In a study by Barnett and Rathmell, Raman peaks of THC were examined by changing the THC concentrations and it was observed that these peaks were observed at 710, 999, 1130, 1232, 1352 and 1390 cm-1. It was also shown that stronger peaks could be obtained at increasing concentrations (28).
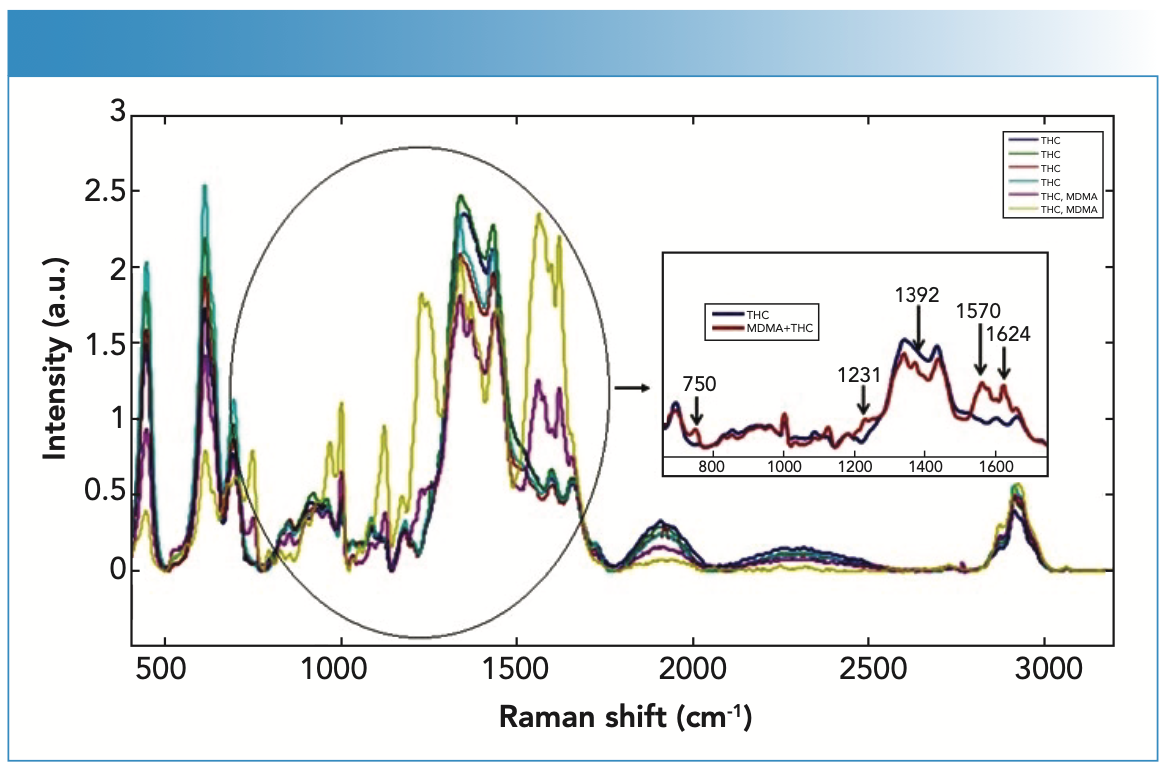
The MDMA peaks differed in a blood sample taken from a person using both MDMA and THC (Figure 4). The most noticeable Raman peaks that distinguished MDMA from THC were found at 750, 1231, 1392, 1570, and 1624 cm-1. These bands can be used to confirm the presence of MDMA. The Raman band at 1624 cm-1 corresponded to vibrations of NH deformation.
FIGURE 4: Raman spectra of the blood samples with THC and both THC and MDMA (different peaks observed in blood with MDMA are indicated by black arrows).
Sagmuller and associates stated that there are peaks of C-H and C-C vibrations belonging to methyl and ethyl groups in the region between 900 and 1650 cm-1 for MDMA analysis (1). Westa and coauthors (9) stated that the most prominent Raman peaks for MDMA were at 716 cm-1 and 810 cm-1. Ryder and associates applied different chemometric analyses by dividing into regions between 450 and 1100 cm-1 by micro-Raman spectroscopy. Four different Raman peaks were observed between 700 cm-1 and 900 cm-1 as MDMA peaks in solid mixtures (29). It was observed that the results obtained from the studies were compatible with our study.
Inscore and coauthors performed a drug substance analysis in saliva with the SERS method in which it was shown that the obtained Raman peaks according to the type of drug used and the varying amount of the same substance were also different in their study (30). In the study, SERS of MDMA was found to be at 530–535, 715–720, 1250, 1365–1370, 1430–1435, 1470–1480, and 1620 cm-1, largely due to the dioxole ring alone or coupled with the phenyl ring (26).
PCA Analysis
PCA, which is used as the most preferred method for qualitative classification, has proven very useful in different studies (21,31,32), as well as in our study, to visualize spectral data and examine possible sample groupings according to spectral characteristics.
When the SERS spectra of the blood samples containing THC and MDMA were examined, distinctive peaks were obtained at 500–1600 cm-1. The PCA method was performed for pattern identification of different types of objects in cluster form among illicit drugs of blood samples. In the PCA model, three different principal components (PCs) with different percentages were obtained. It was observed that the percentages of these variables were 55.77% and 19.68% respectively, and the model was classified depending on two PCs, which would make up 75.45% of the total variance. When PCA was applied to the data sets obtained for the analysis of blood samples containing different drugs, it was observed that the data were clustered differently. The PC1 and PC2 scores interpreted the highest variance, and the plot and loadings of two factors are shown in Figure 5a and Figure 5b. The figure of PC2 versus PC1 was illustrated in Figure 5b. PC1 was the most discriminatory, and illustrated in Figure 5a. The PCs indicated the importance of drugs in a given blood sample. In Figure 5a, PC1, which explains most of the variance, was located on the top as positively and strongly correlated with MDMA.
FIGURE 5: PCA scores plot for the first two components of the spectral variance of eleven blood samples: (a) PC1 was the most discriminatory where PCs indicated the importance of drugs in a given blood sample. PC1, which explains most of the variance, was located on the top as positively and strongly correlated with MDMA; (b) The figure of PC2 versus PC1; (c) Prediction scores for blood samples using PLS-DA model with control group and different drugs loaded as predictions. Dashed line represents the default classification threshold.
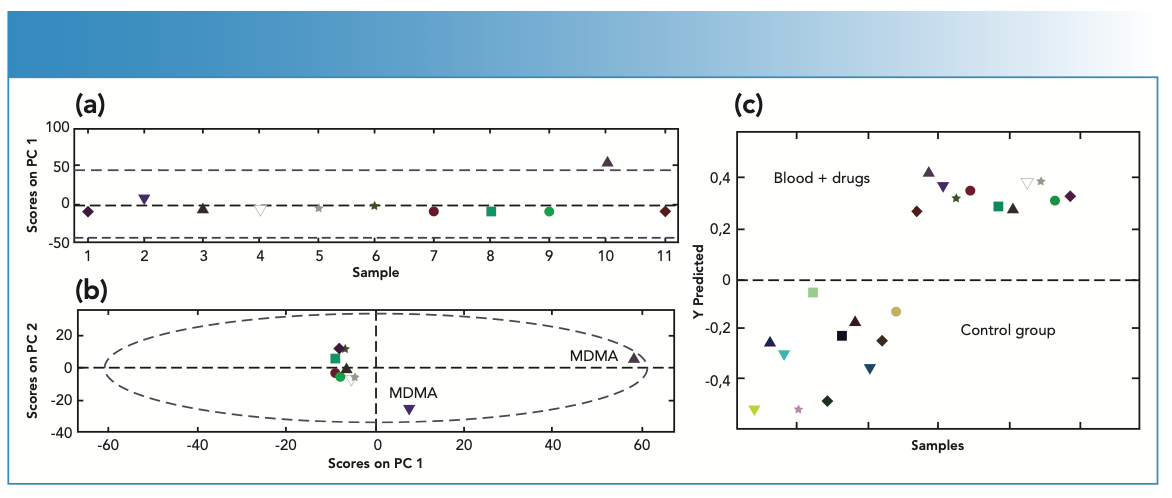
Using a small number of PCs is sufficient, as it uniquely captures the effect of a certain combination of relevant identifiers. For this reason, two PCs were sufficient for the visualization of the investigated blood samples (33). Another preferred reason for the application of PCA to the obtained data from the spectra in the study was that only some components in a mixture were known (18).
PLS-DA Analysis
In this study, since the variability of the data obtained by Raman spectroscopy was high, PLS-DA analysis was applied to determine the variable to be examined. Blood taken from the experimental group (drug users) and the control group (non-drug users) were divided into two groups for PLS-DA analysis. SERS spectra obtained from these two separated groups were transferred into the PLS toolbox as a training and test set. A PLS-DA classification model was built using a training data set containing SERS spectra from the control and experimental groups. The scores obtained in the PLS-DA model were used to evaluate the performance of the model. The leave one out cross-validation (LOOCV) method was used for calibration and validation of the PLS-DA model. Sensitivity, specificity, class error, the root mean square error of calibration (RMSEC), the root mean square error of cross-validation (RM-SECV), and R2 values were calculated to measure the difference. These values were used to describe the performance of the classification model.
RMSEC values were used to differentiate between the reference and the test set. CV values were used for the low number of samples for validation of a classification model, and RMSEC values were also used to evaluate the compatibility of the calibration model with the calibration set (31,34).
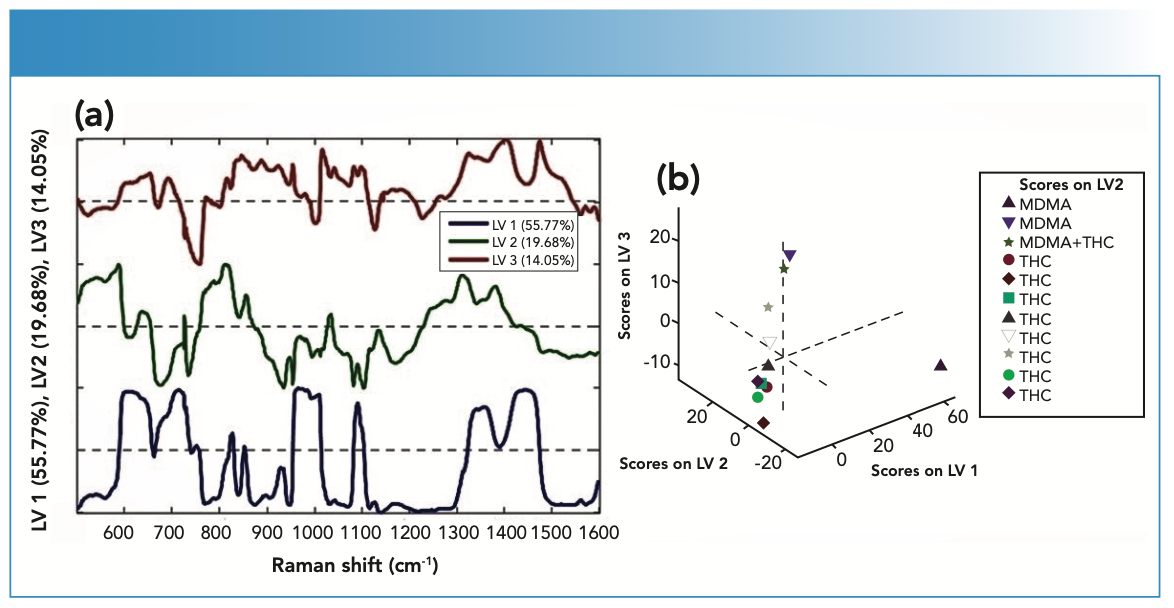
The PLS-DA models gave sensitivity and specificity values on the test set in the range of 88%–100%. The sensitivity values were found to be on the test set in 100%, and specificity values were found to be on the test set in the range of 81.8%-90.9% for the PLS-DA models. RMSEC values were found to be 2% and 3%. RMSECV values were found to be 0.37% and 0.58%. Class error values were found to be between 0 and 9%. The obtained values were found separately as calculated and cross-validated. Obtained scores using the PLS-DA model with the control and experimental groups were loaded as predictions (Figure 5c, and the model was built with three latent variables (LVs). When the loading plots were examined, it was observed that there were significant differences in blood samples, blood samples with THC, and blood samples with MDMA in the region between 500 and 1600 cm-1 (Figure 6a). When the wavelengths of LVs were examined, it was observed that they resembled the spectra obtained from blood samples, blood samples with THC, and blood samples with MDMA (LV1, LV2, and LV3, respectively) and were compatible with the stated respective Raman bands. Scores on LV2 were used to create three-dimensional plots of blood using latent variables (Figure 6b). The LV values obtained in this method allowed for the visualization of different variables. Over-fitting in PLS models reduced the probability of estimation of the results. Therefore, the selection of latent variables was important. Too many latent variables can cause high bias and low variance (35).
FIGURE 6: Loading plots; (a) Latent variables representation with different percentages obtained by the PLS-DA method; (b) Scores on LV2.
Conclusion
The combination of Raman spectroscopy and chemometric techniques allows for the analysis of even the smallest analyte that can be found in biological samples. We have demonstrated an analysis of Raman spectroscopy that is a viable tool for the rapid, non-invasive detection of different illicit drugs in blood samples. It has been observed that the SERS method and chemometric techniques together can be used in drug analysis, even at low concentrations in complex body fluids. RS with PCA and PLS-DA methods of data analysis can be used extensively to build similar or different classification models.
References
(1) Sagmüller, B.; Schwarze, B.; Brehm, G.; Schneider, S. Application of SERS Spectroscopy to the Identification of (3,4-methylenedioxy)amphetamine in Forensic Samples Utilizing Matrix Stabilized Silver Halides. Analyst 2001, 126, 2066–2071. DOI: https://doi.org/10.1039/b105321n
(2) World Drug Report 2020: Drug Use and Health Consequences. (United Nations Publication, Sales No. E.17.XI.6) https://wdr.unodc.org/wdr2020/field/WDR20_Booklet_2.pdf
(3) World Drug Report 2014 (United Nations publication, Sales No. E.14.XI.7) https://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf
(4) Khiabani, H. Z.; Bramness, J. G.; Bjorneboe, A.; Morland, J. Relationship Between THC Concentration in Blood and Impairment in Apprehended Drivers. Traffic Inj. Prev. 2006, 7 (2), 111–116. DOI: https://doi.org/10.1080/15389580600550172
(5) Yayatürk, A. E.; Ertas, H.; Akgür, S. A.; Ertas, F. N. Kötüye Kullanılan Maddelerin Analizi, Akgür, S. A.; Coşkunol, H., Eds. Ege University Press, 2014, pp. 211–236.
(6) Farquharson, S.; Shende, C.; Sengupta, A.; Huang, H.; Inscore, F. Rapid Detection and Identification of Overdose Drugs in Saliva by Surface-Enhanced Raman Scattering Using Fused Gold Colloids. Pharmaceutics 2011, 3, 425–439. DOI: https://doi.org/10.3390/pharmaceutics3030425
(7) An, J. H.; Shin, K.-J.; Yang, W. I.; Lee, H. Y. Body Fluid Identification in Forensics. BMB Rep. 2012, 45 (10), 545–553. DOI: https://doi.org/10.5483/bmbrep.2012.45.10.206
(8) de Oliveira Penido, C. A. F.; Pacheco, M. T. T.; Novotny, E. H.; Lednev, I. K.; Silveira, L. Jr. Quantification of Cocaine in Ternary Mixtures Using Partial Least Squares Regression Applied to Raman and Fourier Transform Infrared Spectroscopy. J. Raman Spectrosc. 2017, 48 (12). DOI: https://doi.org/10.1002/jrs.5231
(9) West, M.J.; Went, M.J. Detection of Drugs of Abuse by Raman Spectroscopy. Drug Test. Anal. 2011, 3, 532–538. DOI: https://doi.org/10.1002/dta.217
(10) Sikirzhytski, V.; Virkler, K.; Lednev, I. K. Discriminant Analysis of Raman Spectra for Body Fluid Identification for Forensic Purposes. Sensors 2010, 10, 2869–2884. DOI: https://doi.org/10.3390/s100402869
(11) Zapata, F.; Gregório, I.; García-Ruiz, C. Body Fluids and Spectroscopic Techniques in Forensics: A Perfect Match? J Forensic Med. 2015, 1. DOI: https://doi.org/10.4172/2472-1026.1000101
(12) Premasiri, W. R.; Lee, J. C.; Ziegler, L. D. Surface-Enhanced Raman Scattering of Whole Human Blood, Blood Plasma, and Red Blood Cells: Cellular Processes and Bioanalytical Sensing. J Phys Chem B. 2012, 116 (31), 9376–9386. DOI: https://doi.org/10.1021/jp304932g
(13) Laserna, J. J. Combining Fingerprinting Capability with Trace Analytical Detection: Surface-Enhanced Raman Spectrometry. Anal. Chim. Acta 1993, 283, 607. DOI: https://doi.org/10.1016/0003-2670(93)85274-N
(14) Moskovits, M. Surface-Enhanced Spectroscopy. Rev. Mod. Phys. 1985, 57, 783. DOI: https://doi.org/10.1103/RevModPhys.57.783
(15) Srajer, J.; Schwaighofer, A.; Nowak, C. Surface-Enhanced Raman Spectroscopy for Biomedical Diagnostics and Imaging. Biomed. Spectrosc. Imaging 2013, 2 (1), 51–71. DOI: https://doi.org/10.3233/BSI-120034
(16) Açikgöz, G.; Hamamci, B. Determination of Ethyl Glucuronide (EtG) in Blood Samples Using Partial Least Squares Discriminant Analysis Applied to Surface-Enhanced Raman Spectroscopy. Vib. Spectrosc. 2020, 106, 103012. DOI: https://doi.org/10.1016/j.vibspec.2019.103012
(17) Guo, S.; Rösch, P.; Popp, J.; Bocklitz, T. Modified PCA and PLS: Towards a Better Classification in Raman Spectroscopy–Based Biological Applications. J. Chemom. 2020, 34, 3202. DOI: 10.1002/cem.3202
(18) Brereton, R. G. Applied Chemometrics for Scientists; John Wiley & Sons Ltd, 2007.
(19) Mobili, P.; Londero, A.; Antoni, G. D.; Gómez-Zavaglia, A. et al. Multivariate Analysis of Raman Spectra Applied to Microbiology: Discrimination of Microorganisms at the Species Level. Revista Mexicana De Física 2010, 56 (5), 378–385.
(20) Ballabio, D.; Consonni, V. Classification Tools in Chemistry. Part 1: Linear Models. PLS-DA. Anal. Methods 2013, 5, 3790. DOI: 10.1039/c3ay40582f
(21) Xu, J. L.; Sun, D. W. in Advanced Technologies for Meat Processing, Toldrá, F. and Leo, M. L. N., Eds., 2nd ed.; Taylor & Francis Group, CRC Press, 2018., pp. 17–81.
(22) Lee, P. C.; Meisel, D. J. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. Phys. Chem. 1982, 86, 3391–3395. DOI: 10.1021/j100214a025
(23) Pérez,A.; Prada, Y. A.; Cabanzo, R.; González, C. I.; Ospino, E. M. Diagnosis of Chagas Disease from Human Blood Serum Using Surface-Enhanced Raman Scattering (SERS) Spectroscopy and Chemometric Methods. Sensing and Bio-Sensing Research 2018, 21, 40–45. DOI: 10.1016/j.sbsr.2018.10.003
(24) Movasaghi, Z.; Rehman, S.; Rehman, I. U.; Raman Spectroscopy of Biological Tissues. Appl. Spec. Rev. 2007, 42, 493–54. DOI: 10.1080/05704920701551530
(25) Rana, V.; Canamares, M. V.; Kubic, T.; Leona, M.; Lombardi, J. R. Surface-Enhanced Raman Spectroscopy for Trace Identification of Controlled Substances: Morphine, Codeine, and Hydrocodone. J. Forensic Sci. 2011, 56(1), 200–207. DOI: 10.1111/j.1556-4029.2010.01562.x
(26) Farquharson, S.; Brouillette, C.; Smith, W.; Shende, C. A Surface-Enhanced Raman Spectral Library of Important Drugs Associated With Point-of-Care and Field Applications. Front. Chem. 2019, 7, 706. DOI: 10.3389/fchem.2019.00706
(27) Yüksel, S.; Schwenke, A. M.; Soliveri, G. et al., Trace Detection of Tetrahydrocannabinol (THC) with a SERS-based Capillary Platform Prepared by the in situ Microwave Synthesis of AgNPs. Anal. Chim. Acta 2016, 939, 93–100. DOI: 10.1016/j.aca.2016.08.033
(28) Barnett N.; Rathmell, C. Detecting Drugs in Saliva. Opt. Photonik 2015, 10 (5), 31–34 (2015). DOI: 10.1002/opph.201500040
(29) Ryder, A. G.; O’Connor, G. M.; Glynn, T. J. Identifications and Quantitative Measurements of Narcotics in Solid Mixtures Using Near-IR Raman Spectroscopy and Multivariate Analysis. J. Forensic Sci. 1999, 44 5), 1013–1019.
(30) Inscore, F.; Shende, C.; Sengupta, A.; Huang, H.; Farquharson, S. Detection of Drugs of Abuse in Saliva by Surface-Enhanced Raman Spectroscopy (SERS). Appl. Spec. 2011, 65 (9), 1004–1008. DOI: 10.1366/11-06310
(31) Westerhuis, J. A.; Hoefsloot, H. C. J.; Smit, S.; Vis, D. J.; Smilde, A. K.; van Velzen, E. J. J.; van Duijnhoven, J. P. M.; van Dorsten, F. A. Assessment of PLSDA Cross Validation. Metabolomics 2008, 4 (1), 81–89. DOI: 10.1007/s11306-007-0099-6
(32) Mazurek, S.; Pichlak, K.; Szostak, R. Quantitative Determination of Vitamins A and E in Ointments Using Raman Spectroscopy. Processes 2021, 9, 8. DOI: 10.3390/pr9010008
(33) Boubchir, M.; Boubchir, R.; Aourag, H. The Principal Component Analysis as a Tool for Predicting the Mechanical Properties of Perovskites and Inverse Perovskites. Chem.Phys. Lett. 2022, 798, 139615. DOI: 10.1016/j.cplett.2022.139615
(34) Rocha, W. F. C.; Prado, C. B.; Blonder, N. Comparison of Chemometric Problems in Food Analysis using Non-Linear Methods. Molecules 2020, 25 (13), 3025. DOI: 10.3390/molecules25133025
(35) Gowen, A. A.; Downey, G.; Esquerre, C.; O’Donnell, C. P. Preventing Over-fitting in PLS Calibration Models of Near-Infrared (NIR) Spectroscopy Data Using Regression Coefficients J. Chemometrics 2011, 25, 375–381 (2011). DOI: 10.1002/cem.1349
Güneş Açıkgöz is with the Vocational School of Health Services at Hatay Mustafa Kemal University, Tayfur Ata Sökmen Campus, in Antakya, Turkey. Abdullah Çolak is with the Department of Biochemistry at Hatay Research and Training Hospital, in Antakya, Turkey. Direct correspondence to: gunesboraacikgoz@gmail.com ●

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.