LIBS Detection of Nanomaterials for Process Control and in the Workplace
Nanostructured materials are expected to lead to the emergence of new products with enhanced functionalities. Their manufacture often requires the use of particles referred to as nano-objects, their aggregates, and their agglomerates. Laser-induced breakdown spectroscopy (LIBS) was deemed as a potential candidate for the detection of these materials in various contexts. This article discusses examples of the application of LIBS for workplace surveillance and process control of nano-objects.
Volume 30 Number 4 Pages 24-33
Nanostructured materials are expected to lead to the emergence of new products with enhanced functionalities. Their manufacture often requires the use of particles referred to as nano-objects, their aggregates, and their agglomerates. Laser-induced breakdown spectroscopy (LIBS) was deemed as a potential candidate for the detection of these materials in various contexts. This article discusses examples of the application of LIBS for workplace surveillance and process control of nano-objects.
Nanotechnology is often said to be the industry of the 21st century. Nanostructured materials are expected to lead to the emergence of new products with enhanced features and functionalities. Their manufacturing often requires the use of nanostructured particles with different sizes, shapes, and chemical compositions, which are referred to as nano-objects, their aggregates, and their agglomerates. These are the basic building blocks used to design nanostructured materials. Carbon nanotubes are often presented as a future superstar in the field of nanotechnology. Their mechanical and electrical properties were demonstrated to be remarkable in comparison with other materials. They are about 100 times more resistant than steel and six times lighter. They are envisaged to reinforce polymers or tires to reduce tire wear. Carbon nanotubes could play an important role in the field of information technology because of their excellent electric conductivity. Nanopowders made of silicon carbide (SiCx) are another telling example. SiCx material is important in the nuclear industry and is a promising material for high temperature applications. Its properties happen to be enhanced when it is prepared using SiCx in a nanostructured form. These are but a few examples of the potential offered by nanostructured materials.
The advent of these new materials raises the question of the development of an adapted metrology for their characterization in various contexts. Two examples of need may be underlined. First, little is known yet as to the risks related to nanomaterials in terms of their impact on human health and the environment. There is therefore a need to detect and characterize these particles in the workplace and elsewhere in the environment. Second, production processes require tools for on-line characterization of the final product. Thus, adapted instruments allowing on-site, on-line, and even real-time determination of the mass concentrations of all the elements the particles are made of must be designed to meet the aforementioned needs. Laser-induced breakdown spectroscopy (LIBS) (1,2) was deemed as a promising technique for this purpose.
LIBS displays advantages of great interest for industrial applications. Detection of all the elements is possible at various pressure conditions. In addition, samples do not need preliminary preparation, which makes LIBS eligible for on-line monitoring. It is a multielemental analysis technique with simultaneous detection of all the elements contained in the sample. Because LIBS is all optical, it is not intrusive and requires only optical access to the sample and thus allows for in-situ measurements. LIBS is potentially fast and suitable for real-time monitoring; its speed depends on the number of laser shots required to obtain reliable results. Remote or stand-off analyses are also possible. The aforementioned features do make LIBS a promising analytical chemistry method for use at industrial sites. Current elemental analysis techniques require time-consuming procedures involving several steps. Skilled personnel are sent to the sites to pick up samples, and then after the samples are back at the laboratory, they must be prepared before they can be analyzed. The LIBS systems developed at the French National Institute for Industrial Safety and Environmental Protection (INERIS) are intended to be operated on-site, in automatic mode, without manual intervention except for maintenance. The LIBS technique may be of use for many applications.
When it comes to LIBS experiments, two important parameters, time delay and integration time, are worth discussing. Indeed, the laser-induced plasma has a transient nature. It is not a continuous plasma discharge in space or in time. The plasma lifetime is a few tens of microseconds and its dimensions are a few millimeters at full expansion. This transient nature has an effect on the detection. To get the best signal-to-noise ratio when recording LIBS spectra, in most cases one must use detectors with a time resolution of a few nanoseconds (such as intensified charge-coupled device [CCD] cameras). During ignition, the plasma is far too luminous for spectra to be recorded. The lines of analytical interest are not visible; they are masked by a strong continuum radiation. Thus, spectra are recorded with a precise time delay and integration time or gate width. The time delay represents the duration elapsed between the firing of the laser and the beginning of plasma spectrum recording. The integration time refers to the duration of the spectrum recording. These two parameters are worth discussing because they will always be found when reading LIBS literature.
As already emphasized above, two examples of LIBS application are presented below. The first is related to process control and the monitoring of SiCx nanopowders while these are being synthesized with a preindustrial pyrolysis reactor. The second has to do with workplace surveillance and detection of carbon nanotube balls while they are being handled. Finally, the coupling of the LIBS technique with a particle trap is presented as a way to improve sampling.
LIBS Experimental Setup
Figure 1 shows a typical LIBS system dedicated to aerosol analysis. Laser pulses with energies of 300 mJ and durations of 6 ns fired by a 1064-nm Q-switched laser (Quantel Brilliant) operating at 20 hz are first spatially expanded to three times the initial beam diameter. With such an increase in radius, the power density deposited on the window when focusing laser pulses inside the cell where the particles are flowed is reduced. A dichroic mirror fully reflective at 1064 nm and 355 nm, but otherwise transparent, directs the beam toward an 85-mm focal length lens, which can focus laser pulses within either the flow cell (workplace surveillance and process monitoring) or the radio frequency plasma discharge (when testing particle trapping). The mirror is positioned so as to make a 90° angle between the optical axis of the beam expander and that of the focusing lens. With such an optical path, light collection along the focusing axis is made possible, given that the dichroic mirror is transparent to almost all wavelengths except for the laser. Light emitted by the plasma is then collected through the same lens used for laser focusing and focused on the entrance of a 600-μm core diameter optical fiber (Ocean Optics) using another 50-mm focal length lens. Light collection may also be effected by using a telescope. The optical fiber is connected to a spectrometer. An Andor Mechelle 5000 was utilized for process control and workplace surveillance whereas an iHR320 (Horiba Jobin Yvon) was used for the experiment with the plasma radio frequency cell. Both spectrometers were equipped with a gated intensified CCD camera (Andor iStar model DH734-18F-03). Time-resolved measurements are made possible with such a detector by triggering signal recording when the laser pulses are fired and then recording the signal over the required duration by selecting the appropriate integration time (with a time resolution of around 2 ns).
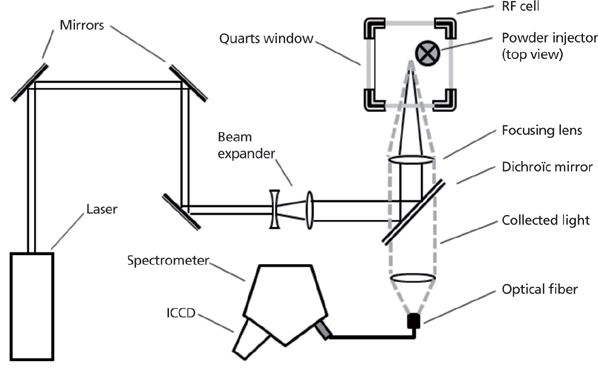
Figure 1: Typical LIBS setup applied to the detection of particles in a radio frequency plage.
Results and Discussion
LIBS Applied to Process Control
With the advent of nanotechnology, production of nanoparticles is a fast expanding industry. Laser pyrolysis is one example of production process aiming at producing nanoparticles (3) with various compositions. The compounds are synthesized by interacting liquid (in aerosol form) or gaseous reactants with a continuous high-power CO2 laser beam. An inert gas such as argon may be used as a background gas. This interaction results in the igniting of a flame. Nanopowders are then formed within the flame and carried away by the background gas toward a particle collector. Though the nanoparticle production processes are intended to be reliable, malfunctions can happen and powder characteristics may not be those expected in the end. Among these characteristics, the correct stoichiometry is important. Until recently, sampling powder after the end of the manufacturing cycle (opening the particle collector) was the only way to measure the relative abundances of all the elements composing the product. Thus, should a disruption occur and the relative composition of the final product not be what was expected, the nanoparticle batch could be lost. This is a real hindrance considering that the cost of such a batch is far from negligible. In this framework, the LIBS technique was seen as a potential candidate for real-time monitoring of the elemental composition of the synthesized product.
Thus, a LIBS system was installed on a laser pyrolysis unit as shown in Figure 2. As already indicated above, the nanopowders in the pyrolysis unit flow through a duct toward the particle collector. They are carried away with argon as the background gas using a pump placed downstream of the collector and connected to an exhaust-treatment system. A metallic flow cell devoted to LIBS analysis through which part of the nanoparticle flow was diverted was branched on the production duct. A bypass line consisting of a 6-mL i.d. stainless steel tube was specially designed to achieve this diversion and enable an on-line continuous sampling from the main stream of nanoparticles. Using the bypass line, the inlet end of the cell was connected to the nanoparticle production conduit upstream of the nanoparticle collector. The outlet end was linked to the exhaust duct downstream of the collector. The preexisting pressure difference between the two connection points suffices to make the nanoparticles carried by the argon gas stream circulate through the cell. A small filter collector was placed downstream of the cell outlet to prevent the particles from being released in the main line. The laser pulses were focused inside the cell right on the particle flow.
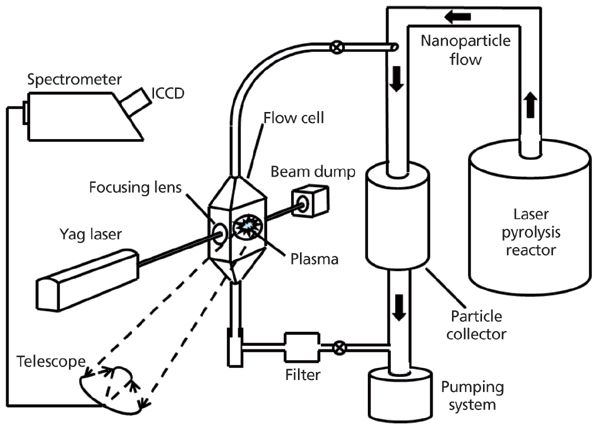
Figure 2: LIBS system setup dedicated to the detection of powders produced by a laser pyrolysis reactor.
Spectra of the silicon and carbon elements were successfully recorded. The relative abundances of each element in the powder were determined without resorting to any calibration procedure. Determination of the relative composition of the powders was achieved assuming that the experimental intensity lines corresponding to the elements of interest could be simulated according to Boltzman's law. This classical statistical physics law applies when the plasma is considered in the local thermodynamic equilibrium (LTE) state. This state stands when collisional processes equilibrate whereas photonic processes do not. The LIBS temporal settings were selected to allow the LTE equilibrium state to stand, and the electronic temperature and density were calculated to check that the equilibrium state was maintained. To make the determination of the relative abundances possible, species densities (for instance Si and C for SiCx) should relate to the corresponding selected lines for each element on the experimental spectrum. Determining the ratio of the densities of each element in the plasma allows for inferring the relative abundances of each element the particles were composed of before their vaporization within the plasma. Eventually, the relative abundances of each element composing SiCx were successfully determined. However, the error margin made when calculating the ratio was assessed at around 20%. These promising results may be improved by simulating synthetic spectra instead of making an analytical calculation. These simulations are currently under way.
LIBS Applied to Workplace Surveillance
The LIBS technique may also be used to make workplaces safer. Carbon nanotube powders manufactured by the Arkema research center present themselves as micrometer-sized balls as may be seen in Figure 3. This conformation is inherent to the production mode in a fluidized bed (4). The issue of workplace safety is raised when these powders are being handled. Do they aerosolize when stirred up, and if so, how can they be detected? Here, again, LIBS was proposed as a tool for real-time detection of possible particle emission.

Figure 3: Examples of carbon nanotube balls.
The handling experiments were carried out in a high safety cell - a room where the carbon nanotubes can be handled safely - by an operator wearing a protective suit for chemical hazards. Three handling scenarios were played out. The first two simulated accidental tipping by pouring one full jar of carbon nanotube powders into another without interruption. In the first scenario, the ventilation was turned on, and for the second it was turned off. The last scenario corresponded to normal working conditions in which jars are filled with powders using a small shovel. In the course of the experiments, LIBS spectra were recorded both before and during handling.
The LIBS system was installed in a room next to the high safety cell along with an aspiration transmission electron microscope (TEM) sampler. The latter instrument allows sampling particles on TEM grids for subsequent particle imagery and energy dispersive X-ray spectroscopy (EDX) analysis. The sampling probes were passed through a plastic window shared by both rooms.
Single-Particle Detection
Real-time detection of carbon nanotube balls requires individual particle analysis, which can be accomplished using LIBS. Indeed, the laser-induced plasma has a transient nature and is not a continuous discharge. It sparks in the air with a frequency matching that of the laser (20 Hz in our experiments). Its lifetime is about a few tens of microseconds and the sampling volume is 10-4–10-3 cm-3.
Thus, when it comes to probing a low concentrated aerosol constituted of micrometer-sized particles, analyte sampling, that is to say plasma particle interaction, becomes a highly stochastic process. This means that single particle detection is possible, which is clearly an advantage for carbon nanotube ball analysis.
Spectra are therefore recorded that correspond to one unique laser shot and only one particle (or carbon nanotube ball) within the laser-induced plasma. A spectrum displaying signals represent a hit or a particle vaporized within the laser-induced plasma. The ratio of the hits to the total number of laser shots is referred to as the sampling rate.
Carbon Nanotube Ball Detection
Thus, the spectra recorded when the carbon nanotube powders were being handled were examined along with those obtained before the handling operations. When handling powders, spectra were recorded that displayed simultaneous detection of aluminum, iron, and carbon nitride. The latter compound originates from the recombination of carbon with nitrogen in the air when the plasma cools down and acts as a marker for carbon detection. Carbon nitride intensity lines were seen more intensely in spectra corresponding to carbon nanotube powder handling than in those registered before handling. Never was such simultaneous detection of these three elements achieved before the handling operations. In addition, the sampling rate was found to be related to the intensity of the handling activity. TEM analysis of the grids revealed that the carbon nanotube balls were exclusively composed of carbon, aluminum, and iron. Other particles such as soot produced along with carbon nanotube balls were not found to contain aluminum or iron. All of these considerations eventually made it clear that the simultaneous detection of aluminum, iron, and carbon nitride lines is the signature of a carbon nanotube ball being detected by the laser-induced plasma (Figure 4). The objective of the next set of experiments was to make these promising results quantitative.
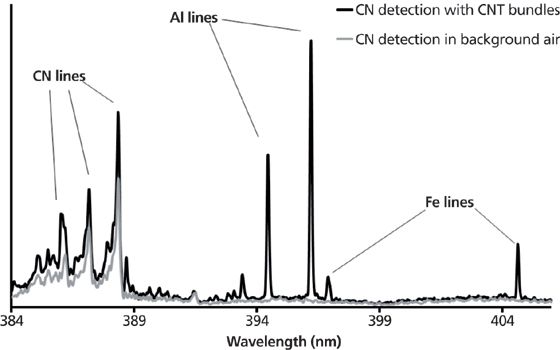
Figure 4: LIBS signature of carbon nanotube balls.
Improving Sampling by Coupling the LIBS System with a Radio Frequency Plasma Discharge
The analysis of particles in a flow cell like that described and presented above raises sampling issues. As emphasized above, the laser-induced plasma has a transient nature and the laser-induced spark is neither continuous in space nor time. In addition, the laser-induced plasma volume is tiny in comparison to the inner volume of the LIBS cell. In other words, many particles of the aerosol flow will escape the analysis. This raises the question of particle sampling improvement. A way to sample particles should be found that allows for analyzing all of the collected particles. Preconcentrating on a substrate or a filter may be a solution. Still, strong matrix effects may appear (5). Another interesting way to deal with such an issue is particle trapping. Such trapping may be achieved using a radio frequency (RF) plasma discharge (6). Thus, the LIBS technique and even other laser diagnostics may be coupled with the plasma discharge, which acts as a particle trap. This coupling presents several advantages and its basic principle is as follows. Particles are injected in the low pressure (1 mbar) RF plasma cell where they remain trapped, frozen in levitation. Particles or nano-objects are therefore accessible to the analysis using laser techniques and even RF plasma diagnostics. Such coupling does present unique features in comparison to current commercially available instruments:
- Particles are trapped within the low-pressure plasma instead of being flowed.
- The RF plasma cell makes the particles levitate within the plasma.
- The particles are almost immobilized or frozen.
- The RF plasma deagglomerates agglomerated particles.
- The RF cell acts as a preconcentrator because particles sampled in the cell cannot escape the analysis and remain detectable as long as the RF discharge is on.
- Particles may be analyzed without using substrates.
- Single-particle analysis is easily made possible.
- Last, but not least, elemental composition determination of organic particles may be facilitated using an inert buffer gas such as argon or helium.
Acting as a preconcentrator, the coupling of laser spectroscopy diagnostics (including LIBS and other diagnostics such as multiangle light scattering) with the RF discharge lets users foresee interesting perspectives for the development of a new "multi-characterization" tool dedicated to ambient air monitoring and process control and allowing access to the following particle features on-line and in real-time:
- Elemental composition,
- Particle size distribution,
- Particle morphology, and
- Particle concentration.
In this context, preliminary experiments were carried out to demonstrate the feasibility of particle detection by LIBS within the low pressure plasma discharge at around 0.25–1 mbar.
Presentation of the RF Plasma Discharge
The RF device presents itself as a 10-cm-wide and 10-cm-high rectangular shaped stainless steel cell. Four optical windows allow implementing optical diagnostics. A pair of 6-cm-diameter parallel disk-shaped electrodes with a 5-cm gap separation is enclosed within the cell in the horizontal position. The electrode from the bottom is connected to the ground, while the top is fed by an RF generator operating at 13.56 MHz. The RF cell is vacuumed using a BOC Edwards XDS-5 pump down to a pressure of 0.25 mbar. Background gas is then flowed in from the top of the cell using a precision valve.
A particle injector positioned on the top of the RF cell was used to flow particles into the low pressure plasma. It consists of a 1-cm-diameter and 0.5-cm-height cylindrical metallic receptacle filled in with powders. The receptacle is capped with a 5-μm step mesh grid facing the inner plasma RF discharge chamber when placed in operational position. Its functioning is close to that of a salt shaker, forcing particles into the RF plasma when the shaking action is electrically activated.
Detection of Particles Coupling LIBS and the Radio Frequency Plasma Discharge
Agglomerates of composite nanoparticles of SiC and Al2O3 with sizes of 300 nm and 1 μm, respectively, were injected into the RF plasma discharge where they were trapped and remained in levitation. The trapping in levitation caused by the two main forces exerted on the particles, namely gravity and electrostatic forces originating from the electric field created by the RF discharge. Both forces happen to balance (6). Nanosecond laser pulses were then focused on the trapped particles.
Two main results were eventually obtained. First, LIBS detection was achieved. Aluminum and silicon lines were detected with a satisfying signal to noise ratio at a reduced pressure of 0.25 mbar instead of the atmospheric pressure. It should be noted that, depending on the particle concentration in the cell, single-particle analysis is made possible. Examples of spectra are presented in Figure 5, with each spectrum corresponding to one unique laser shot.
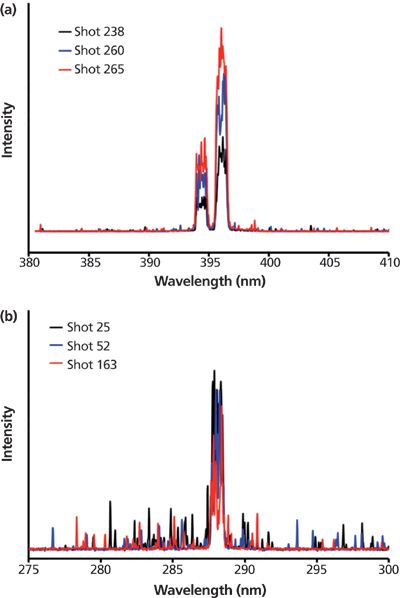
Figure 5: Single-shot detection of (a) aluminum and (b) silicon elements when analyzing particles within the radio frequency plasma discharge.
Second, time delays maximizing the LIBS signal were much shorter than those generally used when analyzing particles at atmospheric pressure. Indeed, contrary to what happens at atmospheric pressure, the microplasma formed on the particles cools much faster at reduced pressure than at atmospheric pressure. As a consequence, time delay that maximizes detection is much shorter than at atmospheric pressure (Figure 6).
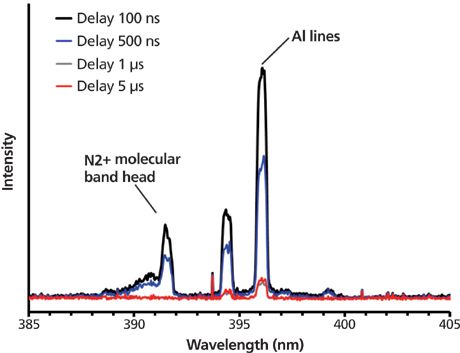
Figure 6: Temporal evolution of the aluminum elements when analyzing alumina particles in the radio frequency plasma discharge.
Conclusion and Perspectives
The LIBS technique has been applied to the detection of nanomaterials for both process control and workplace surveillance. It is seen as a promising tool for both applications. Stoichiometry of composite nanopowders was successfully measured. Emission of carbon nanotube balls was successfully detected as well, though the detection remains qualitative so far. To improve the detection limits when analyzing particles, a radiofrequency plasma discharge has been used as a particle trap.
Particles trapped in levitation were successfully detected. Experiments are currently in progress to measure the stoichiometry of powders of various compositions in aerosol form without resorting to calibration. The experiments involving the coupling of the LIBS system with the radiofrequency plasma discharge will be carried on to explore the potential of this approach.
Acknowledgments
The author would like to thank Elsevier and Springer for permission to reprint Figures 1, 5, and 6 and Figures 3 and 4, respectively.
References
(1) D.W. Hahn and N. Omenetto, Appl. Spectrosc.64, 335A–366A (2010).
(2) D.W. Hahn and N. Omenetto, Appl. Spectrosc.66, 347–473 (2012).
(3) T. Amodeo, C. Dutouquet, F. Tenegal, B. Guizard, H. Maskrot, O. Le Bihan, and E. Frejafon, Spectrochim. Acta, Part B63, 1183–1190 (2008).
(4) B. R'Mili, C. Dutouquet, J.B. Sirven, O. Aguerre-Chariol, and E. Fréjafon, J. Nanopart. Res.13, 563–577 (2011).
(5) C. Dutouquet, G. Gallou, O. Le Bihan, J.B. Sirven, A. Dermigny, B. Torralba, and E. Frejafon, Talanta127, 75–81 (2014).
(6) C. Dutouquet, G. Wattieaux, L. Meyer, E. Frejafon, and L. Boufendi, Spectrochim. Acta, Part B83-84, 14–20 (2013).
Christophe Dutouquet is a research scientist with the French National Institute for Industrial Safety and Environmental Protection (INERIS). He obtained his master's degree in plasma physics from Pierre and Marie Curie University in Paris and his PhD from Orleans University. His PhD was dedicated to laser spectroscopy. As a research scientist with INERIS, his focus is on developing LIBS systems for the detection of aerosols in connection with industrial and environmental issues.

Christophe Dutouquet

Laser Ablation Molecular Isotopic Spectrometry: A New Dimension of LIBS
July 5th 2012Part of a new podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2012 — the Great Scientific Exchange, the North American conference (39th Annual) of FACSS.
New Multi-Spectroscopic System Enhances Cultural Heritage Analysis
April 2nd 2025A new study published in Talanta introduces SYSPECTRAL, a portable multi-spectroscopic system that can conduct non-invasive, in situ chemical analysis of cultural heritage materials by integrating LIBS, LIF, Raman, and reflectance spectroscopy into a single compact device.