LIBS Imaging Is Entering the Clinic as a New Diagnostic Tool
Spectroscopy
Great interest has recently aroused in the study of the dysregulation of chemical elements within tissues. Information about the distribution of elements in biological tissues can contribute to a more complete medical diagnosis, and can guide therapeutic procedures for many pathologies.
Great interest has recently aroused in the study of the dysregulation of chemical elements within tissues (1,2). Information about the distribution of elements in biological tissues can contribute to a more complete medical diagnosis, and can guide therapeutic procedures for many pathologies (3). For a better understanding of the role of metals in biological mechanisms, their identification and relative quantification in their native physiological environment in tissues is always sought. In pathology laboratories, pathologists might opt for an approach called chromogenic detection that requires the use of stains and indicators. However, such methods suffer from several limitations. They require a long preparation time, are time-consuming, have poor sensitivity, only allow one element to be detected at a time, and are limited to certain metals. As a result, these methods are gradually being replaced by more advanced analytical technologies such as transmission electron microscopy combined with energy dispersive X-ray analysis (TEM-EDX), synchrotron radiation microanalysis using X-ray fluorescence (SXRF), or laser-ablation inductively coupled plasma spectrometry–mass spectrometry (LA-ICP-MS). Although these techniques offer high performance in terms of sensitivity or spatial resolution, or both, the complexity of the equipment along with their specific sample preparation requirements make their use difficult for routine medical diagnosis.
For several years, our teams have been collaborating on the development of laser-induced breakdown spectroscopy (LIBS) imaging for biomedical applications (4-6). In LIBS, the laser-induced plasma generated by focusing laser pulses on the surface of the sample allows a specific optical response to be elicited from the elements constituting the sample. This specific response, resulting from the electronic relaxation of atoms and ions excited by the high plasma temperature, is collected and analyzed using an optical spectrometer. Then, the elemental signal is extracted from the recorded spectrum, and elemental maps can be obtained in a pixel-by-pixel manner by scanning over the sample surface (Figure 1). This technology provides significant advantages such as working at atmospheric pressure, high speed of operation (up to 100 Hz), ease of use, and full compatibility with optical microscopy. It is worth mentioning that one constraint when working with medical specimens is that samples are generally fixed with formalin and further embedded in paraffin (FFPE). This FFPE pre-analytical procedure is the current gold standard for processing and archiving of human tissues. From a technical point of view, LIBS analysis of human paraffin-embedded tissues is challenging because it necessitates mastering laser ablation of soft materials (7,8). Recently, we succeeded in adapting our methodology to human biopsies, with multi-elemental imaging studies performed on normal skin and different skin cancers (9) but also on cutaneous granulomas, pigmented lymph nodes, and skin scars (10).
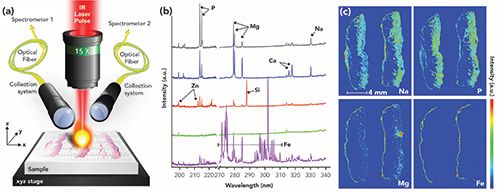
Figure 1: Overview of LIBS imaging technology. (a) Schematic representation of the main components of a LIBS imaging instrument. (b) Typical singleshot spectrum of a tissue biopsy covering 270–340 nm for detecting Mg, Si, Fe, Cu, Al, and Na, and a spectrum covering 190–230 nm detecting P and Zn on different areas of a tissue biopsy. (c) Examples of LIBS images of a paraffin- embedded skin tissue sample.
The achievable performance levels (~ 20 µm resolution, ppm-scale sensitivity, and 100 Hz acquisition rate), its simple instrumentation, and its direct complementarity with histology makes LIBS imaging highly attractive for clinicians as a new diagnostic tool. Accordingly, we are currently working on the translation of this technology to clinical use. In particular, we have invested significant effort in evaluating the feasibility of using LIBS for diagnosis of pulmonary diseases. Indeed, the occupational or environmental inhalation of certain inorganic particles is known to cause various lung diseases that belong to the “pneumoconiosis” spectrum. Etymologically, the word pneumoconiosis comes from the word "konios," which means dust. Diseases belonging to the pneumoconiosis spectrum are silicosis, asbestosis, berylliosis, siderosis, hard-metal lung disease, and so on. In addition, some case reports and preliminary epidemiological data suggest that idiopathic lung diseases (those whose cause is unknown), such as sarcoïdosis or pulmonary fibrosis, might, at least to some extent, be related to inhalation of inorganic particles. For a better understanding of these diseases, clinicians need in situ imaging of metals in human lung biopsies to evaluate whether potential harmful metal elements found in the lungs could relate to a given occupational or environmental exposure.
In the field of respiratory medicine, we have already performed LIBS multi-elemental analysis on several human lung biopsies, after obtaining signed informed consent from every single patient. Clinicians selected lung biopsies from patients with various lung diseases with a potential occupational or environmental origin. We identified several exogenous elements in the lungs of the patients (such as Be, Ti, Si, Al, or Cr), in different areas of the lungs and with various concentration ranges. Importantly, LIBS produced valuable clinical data for most of the patients and elemental findings were generally in accordance with patients’ past exposures. Based on these important preliminary findings, we initiated the first national multicenter retrospective clinical trial aiming at evaluating the feasibility of implementing LIBS imaging as a routine diagnostic test for respiratory diseases (11). This clinical trial involves the participation of five university hospitals in France, and 100 patients will be recruited. The active recruitment of patients is ongoing, and, to date, one fourth of the total cohort has had the elemental content of their lungs analyzed with LIBS. In parallel, we created a control-sample biobank and an associated database through a collaboration with the forensic institute of Grenoble University Hospital. After completing all regulatory and administrative procedures, more than 60 lung specimens from legal autopsies have been collected so far.
In addition, our LIBS multi-elemental analysis has also led to the reclassification of a mislabeled idiopathic lung disease into a compensated occupational disease (emphysema that has been attributed to previous exposure to silica; see Figure 2). To comply with clinical research specifications, the benefit of LIBS imaging in medical diagnosis will only be demonstrated after analysis of hundreds of patients’ normal or diseased lung specimens. Based on serious medical expectations and enthusiasm, but also on our high motivation, such a challenge will require time, staff, and funding.
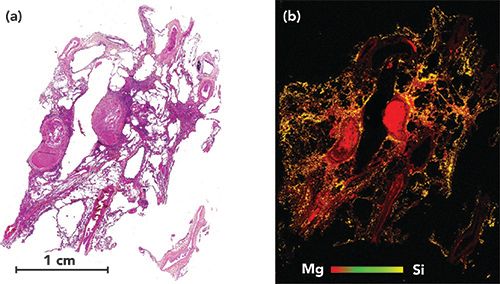
Figure 2: Example of human lung sample analysis, obtained from a patient who underwent lung transplantation for emphysema. (a) Histological image of the lung. (b) Corresponding LIBS multi-elemental images of Si and Mg. In this sample with Mg (red pixels) used as an internal control representing the tissue. This (b) image shows the very high concentration of silica (yellow pixels), in the lung tissue, in a patient who, during her past occupational history, performed sandblasting for one year in very poor conditions.
LIBS is a robust analytical tool, and we strongly believe that it could become a future diagnostic tool providing clinicians with a complementary source of information to better understand the origin and pathogenesis of number of diseases, including respiratory ones.
Acknowledgments
This work was supported by the ITMO Cancer and ITMO Technologies pour la santé de l’alliance nationale pour les sciences de la vie et de la santé (AVIESAN), the Institut National du Cancer (INCa), and INSERM within the project LAST (#PC201513). This work was also supported by the French national grant ANR-17-CE18-0028 “MEDI-LIBS”.
References
- L. Rinaldi, G. Barabino, J.P. Klein, D. Bitounis, J. Pourchez, V. Forest, D. Boudard, L. Leclerc, G. Sarry, X. Roblin, M. Cottier, and J.M. Phelip, Dig. Liver Dis. 47, 602–607 (2015).
- A. Al-Ebraheem, E. Dao, K. Geraki, and M.J. Farquharson, J. Phys. Conf. Ser. 499, 012014 (2014).
- Y. Koga, T. Satoh, K. Kaira, M. Koka, T. Hisada, J. Hirato, B. Altan, M. Yatomi, A. Ono, Y. Kamide, Y. Shimizu, H. Aoki-Saito, H. Tsurumaki, K. Shimizu, A. Mogi, T. Ishizuka, M. Yamada, and K. Dobashi, Environ. Health Prev. Med. 21, 492–500 (2016).
- V. Motto-Ros, L. Sancey, X.C. Wang, Q.L. Ma, F. Lux, X.S. Bai, G. Panczer, O. Tillement, and J. Yu, Spectrochim. Acta B 87, 168–174 (2013).
- L. Sancey, V. Motto-Ros, B. Busser, S. Kotb, J.M. Benoit, A. Piednoir, F. Lux, O. Tillement, G. Panczer, and J. Yu, Sci. Rep. 4, 6065 (2014).
- L. Sancey, S. Kotb, C. Truillet, F. Appaix, A. Marais, E. Thomas, B. van der Sanden, J.P. Klein, B. Laurent, M. Cottier, R. Antoine, P. Dugourd, G. Panczer, F. Lux, P. Perriat, V. Motto-Ros, and O. Tillement, ACS Nano. 9, 2477–2488 (2015).
- B. Busser, S. Moncayo, J.-L. Coll, L. Sancey, and V. Motto-Ros, Coord. Chem. Rev. 358, 70–79 (2018).
- R. Gaudiuso, N. Melikechi, Z. A. Abdel-Salam, M. A. Harith, V. Palleschi, V. Motto-Ros, and B. Busser, Spectrochim. Act. B 152, 123–148 (2019).
- S. Moncayo, F. Trichard, B. Busser, M. Sabatier-Vincent, F. Pelascini, N. Pinel, I. Templier, J. Charles, L. Sancey, and V. Motto-Ros, Spectrochim. Act. B 133, 40–44 (2017).
- B. Busser, S. Moncayo, F. Trichard, V. Bonneterre, N. Pinel, F. Pelascini, P. Dugourd, J.-L. Coll, M. D’Incan, J. Charles, V. Motto-Ros, and L. Sancey, Mod. Pathol. 152, 1–7 (2017).
- The MEDICO-LIBS clinical trial is registered in the clinicaltrials.gov website (NCT03901196).

Benoit Busser is an associate Professor of clinical biochemistry and cancer sciences at CHUGA, the Grenoble Alpes University Hospital, in Grenoble, France, and a senior researcher in the tissue elemental imaging group at IAB, the Institute for Advanced Biosciences (Inserm U 1209/CNRS 5309), at the University Grenoble-Alpes in Grenoble, France.
Vincent Bonneterre is a professor of occupational medicine at CHUGA, the Grenoble Alpes University Hospital, and a researcher at the TIMC-IMAG Laboratory (UMR CNRS 5525), in Grenoble, France.
Lucie Sancey is a CNRS senior researcher at IAB, the Institute for Advanced Biosciences (Inserm U 1209/CNRS 5309) in Grenoble, France.
Vincent Motto-Ros is an associate Professor of Physics at ILM, the Institut Lumière Matière, at the University of Lyon 1-CNRS, in Villeurbanne, France.
Direct correspondence to: bbusser@chu-grenoble.fr

Laser Ablation Molecular Isotopic Spectrometry: A New Dimension of LIBS
July 5th 2012Part of a new podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2012 — the Great Scientific Exchange, the North American conference (39th Annual) of FACSS.
How do Pharmaceutical Laboratories Approach Elemental Impurity Analysis?
May 14th 2025Spectroscopy sat down with James Harrington of Research Triangle Institute (RTI International) in Research Triangle Park, North Carolina, who was the lead author of this study, as well as coauthor Donna Seibert of Kalamazoo, Michigan. In Part I of our conversation with Harrington and Seibert, they discuss the impact of ICH Q3D and United States Pharmacopeia (USP) <232>/<233> guidelines on elemental impurity analysis and how they designed their study.
Developing Sensitive Optical Methods for Early Disease Detection
May 5th 2025Noureddine Melikechi, dean of the Kennedy College of Sciences and professor at the University of Massachusetts Lowell, shares his work on the early detection of diseases like epithelial ovarian cancer and Alzheimer’s.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)