Single-Cell Analysis by ICP-MS—Current Status and Future Trends
Spectroscopy
Single-cell analysis is important in biology and medicine, because it takes into account cell heterogeneity and cellular dynamics, which are governed by cellular crosstalk and the vicinity of cells. Thus, it is of utmost importance to obtain not only information about the heterogeneity of a cell population, but also about their spatial arrangement.
Single-cell analysis is important in biology and medicine, because it takes into account cell heterogeneity and cellular dynamics, which are governed by cellular crosstalk and the vicinity of cells. Thus, it is of utmost importance to obtain not only information about the heterogeneity of a cell population, but also about their spatial arrangement. In the postgenomic area, exciting strategies of single-cell (SC) analysis based on mass spectrometry have emerged, with inductively coupled plasma–mass spectrometry (ICP-MS) playing a central role to address the metallome at single cell level.
Single-Cell Analysis in Solution by ICP-MS
The seeds for this research theme of ICP-MS were already sown more than a decade ago. Indeed, in 2008, Haraguchi and coworkers attempted to measure the metallome of individual cells (1). In this pioneering study, around 70 stable isotopes of different elements were detected in large salmon egg cells (mm diameter). Since then, the technological development has been disruptive, now permitting the measurement of single cells with diameters in the µm range in a high throughput manner. A milestone in this regard has been the introduction of mass cytometry, where metal-labeled antibodies have enabled the measurement of biomolecules (proteins, RNA) in individual cells by ICP-time-of-flight-MS (ICP-TOF–MS). Today, this is the only single-cell mass spectrometry technology mature enough to be applied in large scale multicenter clinical studies. On the other hand, especially when aiming at measuring the metallome (that is, the trace metal content, the accumulation of toxic metals, or metal-based drugs) of single cells, we still encounter technical challenges. Specifically, methodological aspects of measuring cells in suspension that need to be addressed include: (1) true multi-element capability; (2) sample preparation; (3) sample introduction; and (4) cell size determination and quantification strategies (see Figure 1). In the following, we give a brief summary on the state-of-the-art regarding these aspects.
Figure 1: Challenges in ICP-MS–based single-cell analysis by the two complementary workflows: imaging and analysis of cell suspensions.
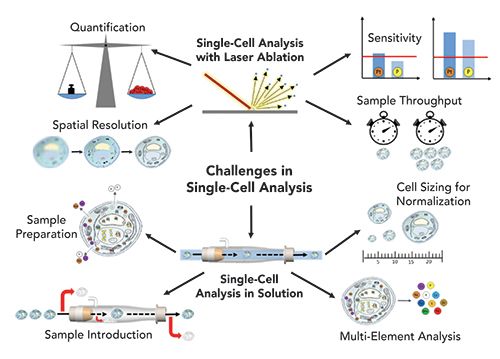
Multi-element analysis. A cell event typically ranges in the time frame of 300 to 500 µs. The settling time of sequential scanning-type ICP-MS (quadrupole or sector-field mass analyzers), which can vary between 0.01 and 5 ms, limits their ability to monitor multiple nuclides during signal pulses of single cells. As a consequence, a number of single-cell studies reported detection of only one element or isotope in the short transient signal of individual cells, which limited the impact of the analysis in the respective field of application (such as toxicity of nanoparticles or metal-based drug uptake) (2,3). To obtain multi-elemental readouts per cell, ICP-TOF–MS instruments are the instruments of choice. These instruments allow the quasi-simultaneous detection of all elements of the periodic table in a single cell (4,5). In mass cytometry, mostly the CyTOF instrument from Fluidigm is used, which is a specialized instrument designed for single-cell analysis and multiplex imaging after antibody labeling that allows the detection of all elements with m/z values between 75 and 209. The icpTOF system from TofWerk AG additionally allows the detection of biologically relevant elements of the lower mass range (m/z = 14–256).
Sample preparation. Prior to analyzing cell samples, typically different sample preparation steps are applied to the sample, including washing, fixation, permeabilization, incubation with drugs, and antibody tagging. All those steps have a potential risk to damage or destroy the cells, leading to a probable loss of noncovalently bound elements in the cells. Up to now, the impact of sample preparation on elements present in the cell has not been comprehensively investigated.
Sample introduction. The term transport efficiency describes the portion of cells entering the plasma. As a major drawback, it is correlated to the size of cells, which makes the unbiased measurement of heterogenous cell populations differing in sizes a challenge. This bias is more pronounced with overall low transport efficiencies. In classical ICP setups, transport efficiencies of only less than 1% could be achieved. Nowadays, transport efficiencies of >50 % are routine (6,7). The improvements were obtained by using full-consumption spray chambers and efficient nebulizers optimized for low flow rates. Finally, autosamplers enable gentle resuspension of cells immediately prior to measurement.
Cell size determination and quantification strategies. Assessing cell size is a prerequisite when comparative elemental single-cell analysis is performed. In fact, the amounts of elements in a cell are regulated by the cell homeostasis mechanisms only in terms of concentration (8). Thus, cell size determination is not only a prerequisite for absolute quantification of intracellular concentrations, but also in the case of multiparametric readouts obtained in mass cytometry, knowledge of cell size is of paramount importance. There are only a few studies existing so far that deal with cell sizing in single-cell ICP-MS (SC-ICP-MS) (9–11). Examples for cell size markers are osmium (or ruthenium) tetroxide or wheat germ agglutinin (WGA). Besides normalization for cell size, standards are required to perform absolute quantification of elements. In SC-ICP-MS, usually liquid elemental standards with a known concentration are used, which are measured equally to the cell samples (12). By taking into account the transport efficiency (determined by polystyrene beads doped with metals or nanoparticle solutions), the measured intensity of a cell event can be extrapolated to the concentration of an element in a single cell. Suitable validation of such quantification exercises is still under debate.
Single-Cell Analysis by LA-ICP-MS
Laser ablation (LA) is a versatile introduction system for ICP-MS and fit-for-purpose for single-cell analysis, because it is characterized by a spatial resolution down to the sub-μm level, limits of detection at the sub-μg/g level, and the ability to selectively target and identify individual cells and components. During the last decade, advances in the development of low-dispersion ablation cells and laminar aerosol transport systems have significantly improved sample throughput and made (intra-)cellular imaging feasible at lateral resolutions on the order of 1 μm (13,14). As a result, the concept of biomarker detection in mass cytometry could be successfully expanded to imaging mass cytometry, making it possible to spatially map the microenvironment of heterogeneous cell populations. In general, single-cell applications by LA-ICP-MS require (a) high spatial resolution, (b) enough sensitivity to detect analytes, (c) high sample throughput and (d) appropriate quantification strategies (see Figure 1). Again, despite exciting progress, there is space for improvement regarding these four key analytical figures of merit to make quantitative, highly resolved elemental bioimaging on single cell level a routine application.
Spatial resolution. Commercially available laser ablation systems provide spot sizes down to 4–1 μm. For imaging mass cytometry, where lanthanide-tagged antibodies are employed to visualize biomarkers, scanning of tissue samples with a laser spot size of 1 μm is standard (15). In a recent report, the features of a low-dispersion ablation cell are described with a spot size of 0.6 μm in diameter on the sample surface, which opens the way for subcellular imaging capabilities (16). Another strategy to improve spatial resolution can be achieved by overlapping spot sizes and line scans and the application of deconvolution approaches (17,18).
Sensitivity. Limits of detection are in the sub-μg level for most elements using LA-ICP-MS; however, there is always a trade-off between spatial resolution and sensitivity. Aiming at single-cell imaging, many studies have focused on the detection of exogenous elements from the higher mass range resulting from treatment with metallodrugs, from nanoparticles or environmental sources. Provided that biologically relevant elements are present at sufficient concentration, they can be detected by LA-ICP-MS at the single-cell level (19).
Sample throughput. Low dispersion LA systems provide pixel-resolved imaging of biological samples of up to 500 kHz compared to a few Hz to 20 Hz, which was the standard of conventional LA systems a few years ago. This significant decrease in analysis time makes it feasible to analyze a sufficient number of cells in a reasonable timeframe (reported numbers are in the range of 500 to 2000 cells per h). Apart from imaging, another approach for single-cell analysis by LA-ICP-MS relies on the quantitative ablation of each individual cell by one laser shot (20,21). In this case, the ablation of one cell takes only 10 to 20 ms; however, the acceleration, travel time, and deceleration of the translation stage limit the speed of analysis, and automatic cell recognition software is still not routinely implemented in LA systems. Bidirectional area scanning can additionally decrease the analysis time significantly, and was used for the three-dimensional mapping of an iridium-intercalator of HeLa cells on single cell level (22).
Quantification strategies. For LA-ICP-MS, accurate quantification is challenged by the limited availability of commercially available and suitable certified reference materials and calibration standards for bioimaging applications. Therefore, quantification approaches are based on matrix-matched standards and in-house developed protocols that include the preparation of tissue-type sections, gelatin-based standards, ink-printed standards, or isotope dilution strategies (23,24). Validation is performed by bulk ICP-MS analysis of the (homogenized) tissue sample or pooled cells after acid-assisted digestion. Other validation strategies rely on a second calibration strategy or on complementary analytical modalities (such as flow cytometry in the case of imaging mass cytometry [25] or synchrotron-based X-ray fluorescence for LA imaging [24]).
Conclusions and Outlook
Technological advancements for single-cell analysis by (LA-)ICP-MS have accelerated in recent years, leading to emerging applications in medicine, biology, environmental research, and drug development. (Imaging) mass cytometry is already a refined single-cell technique showing all required features to be used as clinical diagnostic aid. Additionally, the latest developments have enabled a broader scope of elemental single-cell analysis by (LA-)ICP-MS. Promising proof-of-principle studies have shown applications ranging from nanoparticle accumulation and toxicity studies and metallodrug development to the assessment of the full metallome in a single cell. Despite the fact that most of these studies are currently rather at the idea level of application, we are quite confident that soon technical improvements will enable larger scale biological and biomedical studies.
References
- H. Haraguchi, A. Ishii, T. Hasegawa, H. Matsuura, and T. Umemura, Pure Appl. Chem. 80, 2595–2608 (2008).
- M. Corte Rodríguez, R. Álvarez-Fernández García, E. Blanco, J. Bettmer, and M. Montes Bayón, Anal. Chem. 89, 11491–11497 (2017).
- S. Meyer, A. López-Serrano, H. Mitze, N. Jakubowski, and T. Schwerdtle, Metallomics 10, 73–76 (2018).
- M. Tanner and D. Günther, Anal. Bioanal. Chem. 391, 1211–1220 (2008).
- L. Hendriks, A. Gundlach-Graham, B. Hattendorf, and D. Günther, J. Anal. At. Spectrom. 32, 548–561 (2017).
- A.S. Groombridge, S. Miyashita, S. Fujii, K. Nagasawa, T. Okahashi, M. Ohata, T. Umemura, A. Takatsu, K. Inagaki, and K. Chiba, Anal. Sci. 29, 597–603 (2013).
- H. Wang, M. Wang, B. Wang, L. Zheng, H. Chen, Z. Chai, and W. Feng, Anal. Bioanal. Chem. 409, 1415–1423 (2017).
- M.S. Cyert and C.C. Philpott, Genetics 193, 677–713 (2013).
- M.A. Rapsomaniki, X.-K. Lun, S. Woerner, M. Laumanns, B. Bodenmiller, and M.R. Martínez, Nat. Commun. 9, 632 (2018).
- R. Catena, A. Özcan, N. Zivanovic, and B. Bodenmiller, Cytometry A 89, 491–497 (2016).
- A.D. Stern, A.H. Rahman, and M.R. Birtwistle, Cytometry A 91, 14–24 (2017).
- M. Corte-Rodríguez, E. Blanco-González, J. Bettmer, and M. Montes-Bayón, Anal. Chem. 91, 15532–15538 (2019).
- S.J.M. Van Malderen, A.J. Managh, B.L. Sharp, and F. Vanhaecke, J. Anal. At. Spectrom. 31, 423–439 (2016).
- A. Gundlach-Graham and D. Günther, Anal. Bioanal. Chem. 408, 2687–2695 (2016).
- C. Giesen, H. A. O. Wang, D. Schapiro, N. Zivanovic, A. Jacobs, B. Hattendorf, P.J. Schüffler, D. Grolimund, J.M. Buhmann, S. Brandt, Z. Varga, P.J. Wild, D. Günther, and B. Bodenmiller, Nat. Methods 11, 417–422 (2014).
- S.J.M. Van Malderen, T. Van Acker, and F. Vanhaecke, Anal.Chem. (2020), in press, DOI:10.1021/acs.analchem.9b05056.
- S.J.M. Van Malderen, J.T. van Elteren, and F. Vanhaecke, Anal. Chem. 87, 6125–6132 (2015)
- J. Pisonero, D. Bouzas-Ramos, H. Traub, B. Cappella, C. Álvarez-Llamas, S. Richter, J.C. Mayo, J.M. Costa-Fernandez, N. Bordel, and N. Jakubowski, J. Anal. At. Spectrom. 34, 655–663 (2019).
- S. Theiner, A. Schweikert, S. J. M. Van Malderen, A. Schoeberl, S. Neumayer, P. Jilma, A. Peyrl, and G. Koellensperger, Anal. Chem. 91, 8207–8212 (2019).
- K. Löhr, H. Traub, A.J. Wanka, U. Panne, and N. Jakubowski, J. Anal. At. Spectrom. 33, 1579–1587 (2018).
- K. Löhr, O. Borovinskaya, G. Tourniaire, U. Panne, and N. Jakubowski, Anal. Chem. 91, 11520–11528 (2019).
- S.J.M. Van Malderen, T. Van Acker, B. Laforce, M. De Bruyne, R. de Rycke, T. Asaoka, L. Vincze, and F. Vanhaecke, Anal. Bioanal. Chem. 411, 4849–4859 (2019)
- I. Konz, B. Fernández, M.L. Fernández, R. Pereiro, and A. Sanz-Medel, Anal. Bioanal. Chem. 403, 2113–2125 (2012).
- M. Šala, V. S. Šelih, and J.T. van Elteren, Analyst 142, 3356–3359 (2017).
- T. Van Acker, T. Buckle, S.J.M. Van Malderen, D.M. van Willigen, V. van Unen, F.W.B. van Leeuwen, and F. Vanhaecke, Anal. Chim. Acta 1074, 43–53 (2019).
Gunda Koellensperger

Anna Schoeberl is a doctoral researcher, Sarah Theiner is a postdoctoral researcher, and Gunda Koellensperger is a professor, all in the Institute of Analytical Chemistry in the Faculty of Chemistry at the University of Vienna, in Vienna, Austria. Direct correspondence to gunda.koellensperger@univie.ac.at

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.