Light: Particle or Wave?
The issue of whether light is a particle or a wave was an important historical debate. The actual answer is still unnerving to some people. Here, we will explore the history of this question and present the modern interpretation of the nature of light.

Is light a particle or a wave? Not only was this an important question for the modern understanding of the world around us, but the answer was long in coming.
Particles and Waves
It should be obvious that matter — anything that has mass and takes up space — is ultimately particulate. In fact, matter defines what it is to be a particle. Particles come in pieces that can be broken into smaller pieces, and smaller pieces can be fused together to make larger pieces. No two pieces can occupy the same exact point in space, a concept that applies at the atomic and molecular level for matter that is a solution, a liquid, or a gas. Matter in motion tends to move in a straight line unless some force acts on it, and matter in motion tends to follow Newton's laws of motion (which can be reformulated into Lagrange's or Hamilton's laws of motion). Moving matter can be blocked by the presence of other matter, much like an umbrella blocks the paths of raindrops falling on it. Moving matter also can bounce off of surfaces, as demonstrated by many a basketball player. See Figure 1.

Figure 1: One property of matter is that no two pieces of matter can fit in the same point in space. This momentum-based toy, called Newton's cradle, is based on that property.
Waves behave differently. Waves (mechanical waves, at least) are a regular disturbance of some medium through which the wave travels and transfers energy. The particles of the medium oscillate regularly either in the direction of the wave (longitudinal waves) or perpendicular to the direction of the wave (transverse waves). A particular wave can be described by its wavelength, the distance between two similar amplitudes of consecutive waves, and its frequency, the number of waves passing a fixed point in space per second. The product of the wave's frequency and wavelength is the wave's velocity. Waves can combine, a process called interference. If similar amplitudes of two waves combine to make a larger wave, the waves are constructively interfering. If opposite amplitudes of two waves combine to cancel each other out, the waves are destructively interfering. Waves can diffract: they can change their direction in their medium, which is why you can hear someone yelling around a corner. Waves can be reflected from surfaces (think of an echo you might hear off of a far building). See Figure 2.

Figure 2: Waves have certain properties that matter does not: wavelength, frequency, interference, and other properties. Permission to reprint courtesy Dr. Andrew Davidhazy, School of Photographic Arts and Sciences, Rochester Institute of Technology.
What is Light?
Is light a particle or a wave? The actual nature of light is a topic that has occupied minds for millennia. The earliest mention of the nature of light might have been by Pythagorus (of Pythagorean theorem fame; ca. 580–500 B.C.), whose particle hypothesis of light suggested that light particles were emitted by all objects and were intercepted by the eyes (called the "intromission hypothesis" of light). However, Plato (ca. 427–347 B.C.) suggested that light was created by the eye (the "emission hypothesis" of light) and illuminated other objects. In contrast, Plato's pupil Aristotle (384–322 B.C.) thought that light was some kind of wave. In about 300 B.C., the mathematician Euclid noted that light traveled in straight lines and obeyed a law of reflection, the concept that the angle of reflection equals the angle of incidence. Euclid also thought that light was a wave that was emitted by the eye, and light waves must move extremely fast because when you open your eyes, objects — even faraway objects — are immediately visible. (The determination of the speed of light is another fascinating story, but that's another column.) In the 10th century A.D., Persian scientist Alhazen Abu Ali al-Hasan Ibn Al-Haitham used experiments to support the intromission hypothesis of light, but apparently did not address the "particle versus wave" nature of light.
The issue of particle versus wave came to a crescendo during the European Renaissance. In 1621, Dutch physicist Willebrod Snell announced Snell's law of refraction, suggesting that light is a wave. A wave nature of light also was espoused by English physicist Robert Hooke, and in 1690, Dutch scientist Christiaan Huygens (Figure 3) published Traite de Lumiere ("Treatise on Light"), which not only supported the concept of light as a wave, but used the wave nature of light to explain many of its properties, including refraction and polarization (a property of light originally discovered by Danish scientist Rasmus Bartholin in 1669). It was the most comprehensive wave description of light to date.

Figure 3: Dutch scientist Christiaan Huygens (1629â1695) was an early proponent of light existing as a wave.
Isaac Newton (Figure 4), however, was impressed by the fact that light casts sharp shadows, as if it were a stream of particles being blocked. He favored a corpuscular nature of light, which he also used to explain reflection and, albeit with difficulty, refraction. Such was Newton's fame that by virtue of his influence, the true nature of light was still uncertain until the early 1800s, although many people were convinced that Newton was correct.

Figure 4: English polymath Isaac Newton (1643â1727) argued that light was a particle. Given his scientific stature, his viewpoint won many converts.
What Light Is — Part I
In 1801, English scientist Thomas Young performed what became known as the double-slit experiment. If a beam of light were passed through a narrow slit and projected onto a screen, a single image would appear, getting less intense as one moved laterally away from the point directly opposite the slit (Figure 5). What would happen if there were two closely spaced slits? If light were a particle, one would see two closely spaced images on the screen.
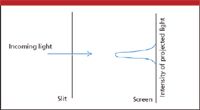
Figure 5: If light is passed through a single slit, a single image with the illustrated intensity profile will appear on a screen. This is what would be expected if light were either a particle or a wave.
However, that is not what Young observed. (Young actually used a thin card to split a narrow beam of light to simulate two closely spaced slits.) Young saw an intense image at a point between the two slits, then dark fringes on either side, then more bright images, then dark images, and so forth (Figure 6). In short, Young was observing constructive and destructive interference. Matter certainly does not constructively and destructively interfere (right?), so light must be a wave. Similar work by Augustin Fresnel and, ultimately, James Clerk Maxwell's 1864 summary of the four equations of electromagnetism (Maxwell's equations) cemented light's nature as a wave.
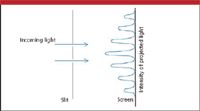
Figure 6: Light passed through two closely spaced slits shows the illustrated intensity pattern on a screen. This pattern indicates constructive and destructive interference, and (supposedly) demonstrates that light is a wave.
What Light Is — Part II
But wait, there's more. In the late 1800s, researchers noted certain behaviors of light (blackbody light, in particular) that did not conform to the currently accepted understanding of nature. Such behaviors included the intensity distribution of emitted light versus wavelength and temperature; variation of emitted wavelength maxima with temperature; and total power emitted. Attempts to explain the behavior of light theoretically ultimately failed. Then, in 1900, German thermodynamicist Max Planck proposed that light emitted from blackbodies originated from oscillators in matter whose energies E were proportional to the frequencies of their oscillations; the modern symbol for the proportionality constant is h, and is called Planck's constant:
E = hν
where ν, the Greek letter nu, is used to represent frequency. Because these matter oscillators are limited to having a certain quantity of energy, they are said to be quantized. After making this assumption, Planck was able to use statistical thermodynamical arguments to derive an equation that fit the intensity distribution of blackbody radiation. Although there was now a theoretical basis for the behavior of light, even Planck was on record as being uncertain about quantized energy of his oscillators.
However, in 1905, German physicist Albert Einstein, a Swiss patent examiner at the time, used Planck's "E = hν" equation and applied it to light itself, suggesting that light had an amount of energy proportional to its frequency. With that interpretation of light, Einstein was able to explain the photoelectric effect, an effect in which electrons were emitted from metals when light was shined on them. The photoelectric effect was another phenomenon that previous science was unable to explain, but Einstein showed that if the energy of light were indeed quantized, the photoelectric effect had a straightforward explanation. Thus, Einstein was able to rationalize Planck's proposal using a completely different phenomenon.
(Science now recognizes that Planck's original assertion was so groundbreaking that science is broken historically into two time periods: pre-1900, or Classical Physics, and post-1900, Modern Physics.)
Einstein's interpretation of light, then, is that it acts like a particle of energy; these particles were eventually named photons. Later, in 1922, American physicist Arthur Compton demonstrated that photons also have momentum, another particle property. It has now been firmly established that light has particle properties, re-awakening the earlier arguments of light as a particle.
So is light a particle or a wave? It has properties of both. Light can be labeled with a wavelength, frequency, velocity; it can reflect, refract, interfere, and diffract. In those (and other) respects, light behaves like a wave. But light also has a certain amount of energy depending upon its frequency, and it also has momentum. In those respects, it acts like a particle.
Some speak of a "particle-wave duality"; others even use a term I shudder at: "wavicle." Perhaps the best perspective is to understand that being a particle or a wave is no longer mutually exclusive. This was reinforced in the mid-1920s, when the modern theory of electron behavior — quantum mechanics — was based upon these tiny pieces of matter acting as waves.
David W. Ball is a professor of chemistry at Cleveland State University in Ohio. Many of his "Baseline" columns have been reprinted in book form by SPIE Press as The Basics of Spectroscopy, available through the SPIE Web Bookstore at www.spie.org. His most recent book, Field Guide to Spectroscopy (published in May 2006), is available from SPIE Press. He can be reached at d.ball@csuohio.edu his webiste is academic.csuohio.edu/ball

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.
Smarter Sensors, Cleaner Earth Using AI and IoT for Pollution Monitoring
April 22nd 2025A global research team has detailed how smart sensors, artificial intelligence (AI), machine learning, and Internet of Things (IoT) technologies are transforming the detection and management of environmental pollutants. Their comprehensive review highlights how spectroscopy and sensor networks are now key tools in real-time pollution tracking.