Meeting the Surge in Demand for Seafood Screening on the Gulf Coast
Special Issues
The authors look at the use of QuEChERS in the ongoing testing program in the gulf.
The recent Deepwater Horizon oil spill in the Gulf of Mexico has raised serious concerns about the effects of petroleum on marine life and humans due to the contamination of one of the richest seafood grounds in the world. Dispersed and dissolved oil in seawater can result in exposure of aquatic resources to the toxicological effects of polycyclic aromatic hydrocarbons (PAHs). As lipophilic compounds, they can cross lipid membranes easily and bioaccumulate in aquatic organisms. PAHs can cause direct toxicity (mortality) to marine mammals, fish, and aquatic invertebrates through smothering and other physical and chemical mechanisms (1). Besides direct mortality, PAHs can cause sublethal effects such as DNA damage, liver disease, cancer, and reproductive, developmental, and immune system impairment in fish and other organisms. PAHs can accumulate in invertebrates, which might be unable to metabolize the compounds efficiently, and be passed up the food chain, ultimately to humans.
The National Oceanic and Atmospheric Administration (NOAA) and FDA are responsible for ensuring the safety of seafood taken from the areas affected by the oil spill. Crude oils differ in how they behave in the environment and how they affect fish and shellfish (2). Where oil is spilled also makes a difference. After a spill, the type of oil and the location of the spill determine which fish or shellfish species are most likely to be exposed to the oil. Oils that mix with the water and spills that occur in deep water are more likely to affect finfish, while heavier oils on shorelines are more likely to affect shellfish, especially bivalve mollusks like mussels.
The next step is to collect seafood samples from the spill area and determine the levels of contamination.
NOAA uses two levels of testing to determine the extent of oil pollution of seafood. The first is a sensory analysis by trained technicians, examining raw sample odor, cooked odor, and cooked taste. If the samples are found to be free of oil "taint" in the sensory test, they are analyzed for PAH content. If PAH levels are found to be lower than established FDA Levels of Concern (LOCs), the fishery can be reopened.
Most seafood risk assessments conducted after oil spills in the U.S. have followed an approach used by the FDA in 1990 after the Exxon Valdez oil spill in Prince William Sound, Alaska, and the procedure has recently been updated (3). It uses a set of calculations to determine maximum PAH tissue concentrations in seafood harvested for human consumption, above which an appropriate, conservatively estimatedupper-bound risk level for cancer is exceeded. Levels of concern for noncancer risks from toxicity are also evaluated. The values for several variables in these calculations can be adjusted on a case-by-case basis, depending on seafood consumption rates of the exposed population, average body weight of the exposed population, estimates of exposure time for a particular spill, and the cancer risk level deemed appropriate. The LOCs that have been set for the ongoing testing after the Deepwater Horizon spill are shown in Table I.
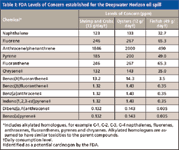
Table I: FDA Levels of Concern established for the Deepwater Horizon oil spill
NOAA uses an established method for sample extraction, cleanup, and analysis by gas chromatography–mass spectrometry (GC–MS) for the analysis of sediment and tissue samples to determine the nanograms-per-gram concentrations of selected aromatic hydrocarbons and chlorinated hydrocarbons (2). The method is designed to monitor the levels of 29 PAHs, and in the case of the Deepwater Horizon spill, the focus is on the 12 compounds shown in Table I. However, the NOAA method is fairly time consuming, taking more than a full day in the laboratory to complete. As a result, most analytical laboratories can only process 30–40 samples per week. Given the enormous number of samples that will have to be tested to ensure the safety of all of the fisheries affected in the gulf region, the established method might not be able to provide the throughput levels required to respond to this crisis in a timely manner.
Speed is Essential
The most time-consuming part of the existing NOAA method is the sample preparation, which involves two clean-up steps and a concentration step to generate a fraction ready for GC–MS analysis. The process is complex, time-consuming, and requires overnight to complete. Therefore, the existing method does not support the throughput required to meet the testing load posed by the Deepwater Horizon spill.
An alternative, much faster method was developed originally to assay pesticides in a wide array of fruit and vegetable matrices. It has been so successful in providing reliable data on a very large number of pesticides in foods that its use has been extended to other compounds in other complex matrices, including the analysis of PAHs in seafood contaminated with petroleum.
QuEChERS to the Rescue
The method is called QuEChERS, which is a portmanteau word formed from quick, easy, cheap, effective, rugged, and safe. Originally developed by Steven J. Lehotay and Michelangelo Anastassiades (4), the method greatly reduces materials costs and combines several different steps, resulting in less chance for error and much shorter preparation times, while also reducing the amount and toxicity of required solvents. Key to the approach is a rapid procedure based upon extraction–partitioning and dispersive solid-phase extraction (d-SPE), which quickly removes water and matrix interferences through the addition of appropriate salts, primary secondary amine sorbent (PSA), and endcapped C18 (C18EC).
For analysis of PAHs, the QuEChERS method starts with chopping and grinding of the seafood sample, usually in frozen form. Acetonitrile and any required internal standards are then added to a weighed amount of sample. Salts are added to promote partitioning of the PAHs from fish matrix and water in the sample into the acetonitrile layer. After centrifugation, the acetonitrile layer is transferred to another tube containing d-SPE sorbent, which removes interferences and fats in the sample matrix.
The ease of use and speed of the QuEChERS methodology for PAH analysis in oil-contaminated samples is enhanced by the availability of kits that have been optimized for a variety of sample types. Extraction kits containing the optimal amount of salts based upon sample amount and tubes containing preweighed d-SPE sorbent (d-SPE kits) eliminate the need for the analyst to manipulate these materials. The end result is a 10-min sample preparation in contrast to the overnight procedure utilized by the NOAA method.
Complete Solutions
The throughput demand caused by the Deepwater Horizon oil spill calls for preconfigured, easy-to-use analytical instrumentation that is optimized for PAH analysis in terms of GC, MS detection, and data analysis. Such systems are now available that are compatible with QuEChERS method kits (Figure 1). They include a GC system equipped with a multimode inlet, integrated capillary flow backflush, and an optimized PAH analysis GC column. Detection is accomplished through either a single-quadrupole mass selective detector (MSD) with preloaded selective ion monitoring (SIM) acquisition and analysis methods, or a triple quadrupole GC–MS system with an optimized and preloaded PAH selective reaction monitoring (SRM) acquisition method and quantification method. Calibration standards and internal standards (ISTDs) for PAH analysis are included along with factory checkout data files.
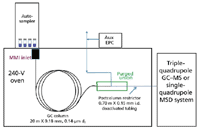
Figure 1: Schematic representation of the preconfigured PAH analysis system, equipped with an optimized GC column, a multimode inlet (MMI), and capillary flow technology backflushing.
Preconfigured PAH analyzers utilize backflush with capillary flow technology to shorten GC run cycle times significantly and eliminate the need for column baking. High boiling hydrocarbons are not transferred to the detector and degradation of the analytical column by "permanently" absorbed components and high-temperature hold times is decreased. Compounds eluted at high temperature are not baked onto detector surfaces, reducing the need for MS source cleaning and maintenance. Capillary flow technology utilizing a unique purged ultimate union provides easier and faster backflushing, with better thermal control and less maintenance.
The use of an auxiliary electronic pneumatic control (EPC) module provides a precisely controlled second source of carrier gas that directs the column flow to the mass spectrometer. In normal operation, the EPC pressure is slightly above the pressure of the carrier gas through the column. During backflush, the column flow is reversed and exits to the multimode inlet split vent. Typically, the reverse column flow is approximately -1.5 mL/min.
High Performance PAH Analysis
Both of these preconfigured systems can resolve the 29 compounds on the NOAA list, with run times under 20 min, as shown on the mass-selective detector system in Figure 2. Note that optimal PAH analyses are run at high temperatures, and in this case the inlet temperature is at 320 °C, the detector transfer line is at 320 °C, the detector source is at 350 °C ( to minimize tailing), and the MS quadrupole is at 200 °C. Backflushing enables analysis of all 29 PAHs in 18 min, plus a 4-min backflush, versus a 53-min run time (excluding necessary bake out time) with the NOAA method.
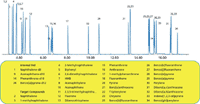
Figure 2: GCâMS analysis of PAH standards at 300 ppb for the 29 compounds listed by NOAA, using the Agilent 7890 GC and 5975C MSD systems in electron ionization (EI) mode, equipped with capillary flow technology backflushing.
Linearity of detection is also excellent with both systems, as shown in Table II. Both the triple-quadrupole (GC–MS-MS) and the MSD (GC–MS) systems give R2 values very close to 1 when the NOAA standards are analyzed in isooctane (first and third columns). Spiking the standards into QuEChERS extract of fish to construct calibration curves resulted in R2 values (middle column) on the triple-quadrupole system that were only slightly lower than those that were obtained in isooctane.

Table II: R2 values from PAH calibration curves*
Recovery values for both systems are also very good, using the QuEChERS extraction method and kits (Table III). Spiking NOAA standards into three separate samples of mussel tissue to give a final concentration of 25 ppb in the extract provides an overall average recovery of 96% for 15 compounds, using either the MSD system or the triple-quadrupole system. Signal-to-noise ratios (S/N) are also similar for the two systems when analyzing PAH standards at 1 ppb in solvent. However, the superior selectivity of MS-MS systems is highlighted by the fact that the S/N is often significantly better using SRM with the triple-quadrupole system (GC–MS-MS), when the standards are spiked into QuEChERS sample extract (Table IV).
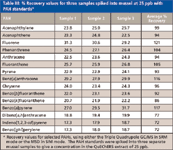
Table III: % Recovery values for three samples spiked into mussel at 25 ppb with PAH standards*
The triple-quadrupole system in SRM mode also has an advantage over the MSD system in SIM mode upon concentrating samples that contain low parts-per-billion levels of PAHs. Detection of very low levels in QuEChERS extracts can require concentration of the extract by as much as 10-fold. This results in background (matrix interference) that is also 10 times as high. When extracts containing 1–6 pg of benzo[b,k,j]fluoranthene were concentrated 10-fold and analyzed on the triple-quadrupole GC–MS system, the SRM qualifyingratios and retention times matched those expected. However, the MSD system in SIM mode did not give ion ratios or retention times that matched the expected when analyzing the 10× concentrate. The triple-quadrupole system can easily detect five of the more toxic PAHs spiked into clean sole, clam, and scallop samples at 67 ppb when extracted using QuEChERS.
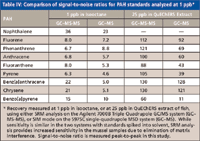
Table IV: Comparison of signal-to-noise ratios for PAH standards analyzed at 1 ppb*
Conclusion
The recent Deepwater Horizon oil spill has highlighted a need for high throughput analysis of PAHs in food and environmental samples. Traditional sample preparation methods are too slow to meet this need. Applying the simple and fast QuEChERS sample preparation method to seafood samples can enable a laboratory to process up to 60 samples a day, versus the 30–40 a week possible with traditional methods. Linearity and recovery of spiked PAH standards are excellent using the QuEChERS method. Although QuEChERS has not yet been validated for PAH analysis in seafood, several laboratories are currently in the process of doing so. To meet the enormous demand for testing that will be generated by the Deepwater Horizon oil spill, QuEChERS could be used as a screening method to identify samples rapidly with PAH levels above the LOCs. The traditional GC–MS method could then be used to confirm the positive findings.
A preconfigured analyzer, combined with use of the QuEChERS sample preparation method, can enable a laboratory to begin sensitive and accurate analysis of PAHs in sea life very quickly, or rapidly expand its current capability. Backflushing is a critical component of such a system as it reduces cycle time and instrument maintenance. While such systems can utilize either triple-quadrupole GC–MS or single-quadrupole MSD instruments, SRM analysis on the triple-quadrupole system can offer lower detection limits for PAHs in complex sample matrix.
Joan Stevens and Mike Szelewski are with Agilent Technologies, Wilmington, Delaware.
References
(1) NOAA Fact Sheet "Shorelines and Coastal Habitats in the Gulf of Mexico," April 2010.
(2) C.A. Sloan, D.W. Brown, R.W. Pearce, R.H. Boyer, J.L. Bolton, D.G. Burrows, D.P. Herman, and M.M. Krahn, "Extraction, cleanup, and gas chromatography/mass spectrometry analysis of sediments and tissues for organic contaminants," U.S. Dept. Commer., NOAA Tech. Memo. NMFS-NWFSC-59, 47 p. (2004).
(3) Laboratory Information Bulletin, FDA/ORA/DFS, version 7/26/2010, www.fda.gov/downloads/ScienceResearch/UCM220209.pdf.
(4) M. Anastassiades et al., J AOAC Int. 86, 412–431 (2003).

Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.